
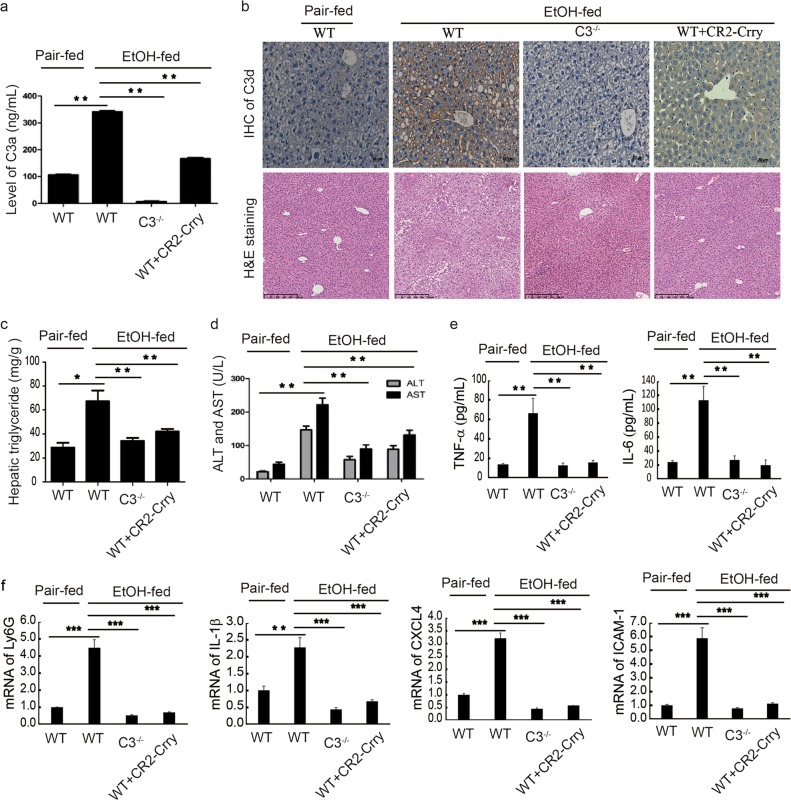
Figure 1 C3敲除后可以治疗酒精性脂肪肝
1a 在减少C3方面使用C3基因敲除鼠和CR2-Crry(一种抑制C3活化的多肽)进行干预,检测血浆中C3a的含量来判断抑制效果
1b-1d 酒精性脂肪肝对应的表型检测
1e-1f 分别检测了血浆中TNFa和IL-6的含量以及肝脏中炎症因子得含量mRNA
CR2-Crry的文献
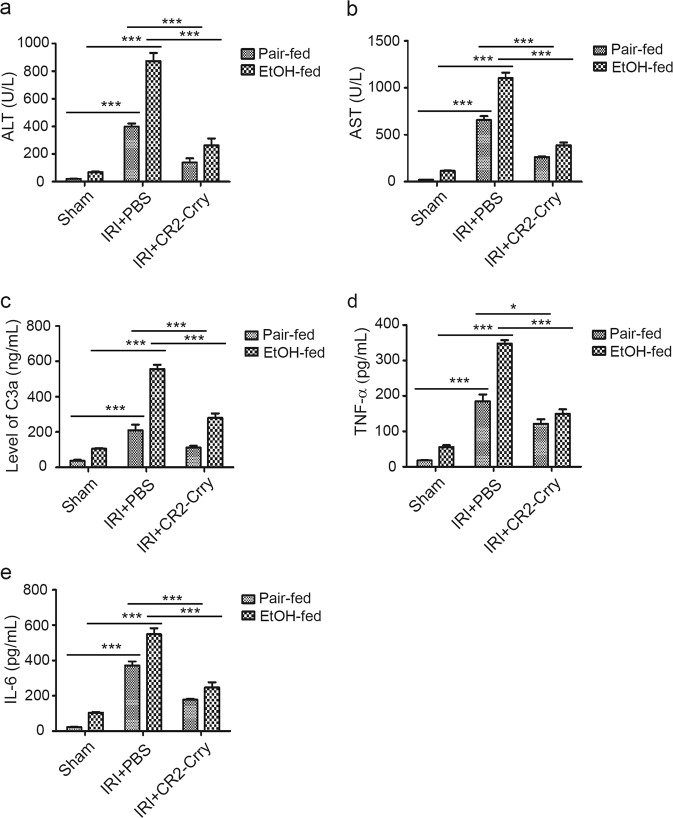
Figure 2 C3缺乏可以治疗肝脏缺血再灌注损伤
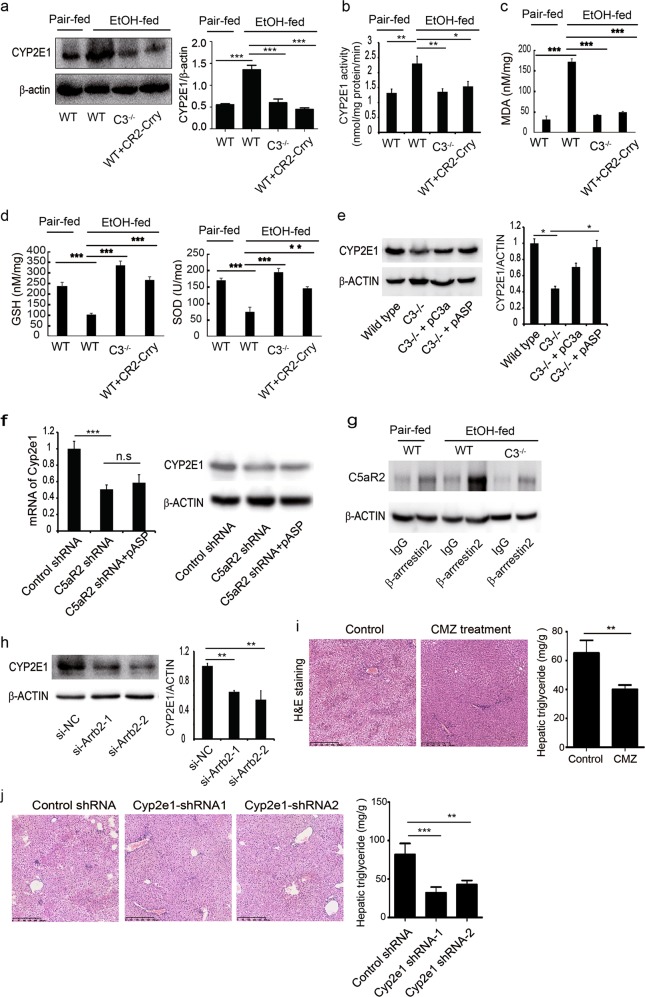
Figure 3 C3的产物ASP与C5aR2结合,进而C5aR2与β-arrestin2结合增加CYP2E1的表达-这里因果关系论证的很好
3a-3d 检测关键代谢酶CYP2E1是否受到影响,氧化应激是否改变
3e 回复实验,证明是C3导致了CYP2E1的改变,因果关系
The decreased expression of CYP2E1 in C3−/− mice was restored by treatment with the peptide C3a or its degraded form, C3a-des-Arg (also known as Asp), again indicating a causative link with C3 activation
3f 还是回复实验,ASP的作用是通过C5aR2
C5aR2 is the only identified receptor for Asp.
3g-3h 原来报道过的分子,β-arrestin2,动物水平上的IP实验和细胞敲除实验
3i-3j 还是用一点实验验证了一下原来报道过的CYP2E1分子的重要性
这里用了AAV9和药物两种方式
既往文献报道:Oxidative stress is a predominant factor that contributes to the pathogenesis of AFLD
细胞色素P450 2E1(cytochrome P450 2E1,CYP2E1)是一种主要位于肝细胞的化合物和毒物的代谢酶,是CYP450代谢酶系家族中的重要一员。它在酒精、四氯化碳、硫代乙酰胺、对乙酰氨基酚、二甲基亚硝胺等毒性底物的代谢和活化中起着重要作用[2-3]。诱导因子作用下,CYP2E1在氧化应激、活性氧簇产生以及酒精性肝损伤增强中可能是一个中心通路。
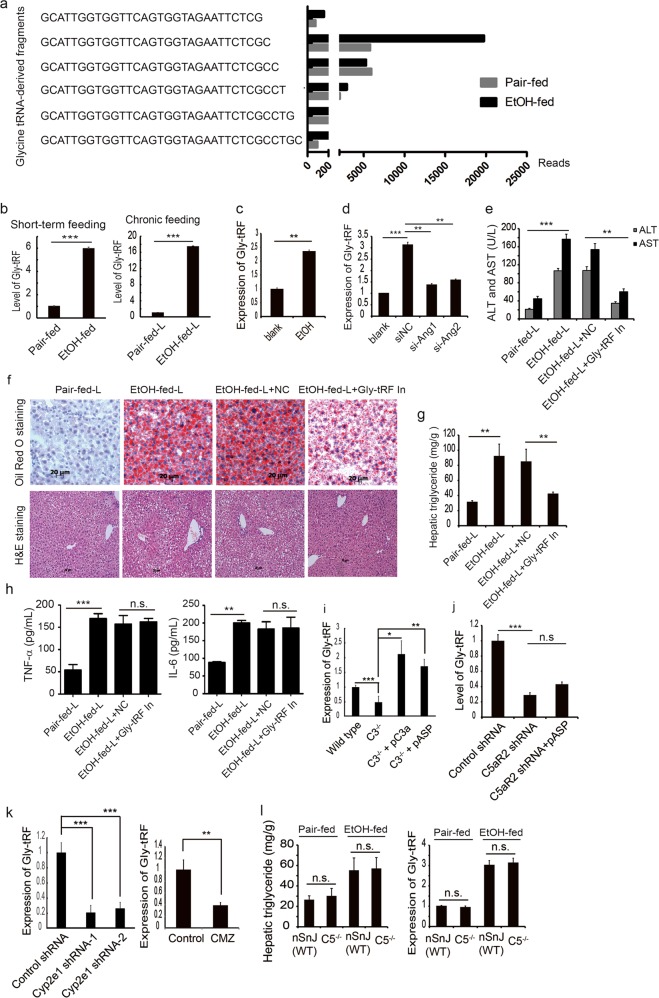
Figure 4 Gly-tRFs是引起酒精性脂肪肝的重要tRNA
4a-4c 找到关键的 tRNA Gly-tRFs-水平改变
4d Several studies have indicated that ANG induced by stress is involved in the biogenesis of tRF
ANG的改变引起的Gly-tRFs-水平(这个就是要与既往研究一致)
4e-4h Gly-tRFs抑制可以治疗酒精性脂肪肝
4i-4k 上述提及的分子均在Gly-tRFs上游,并具有因果关系
4l C5敲除并不影响Gly-tRFs的表达(为什么要加C5敲除鼠?)
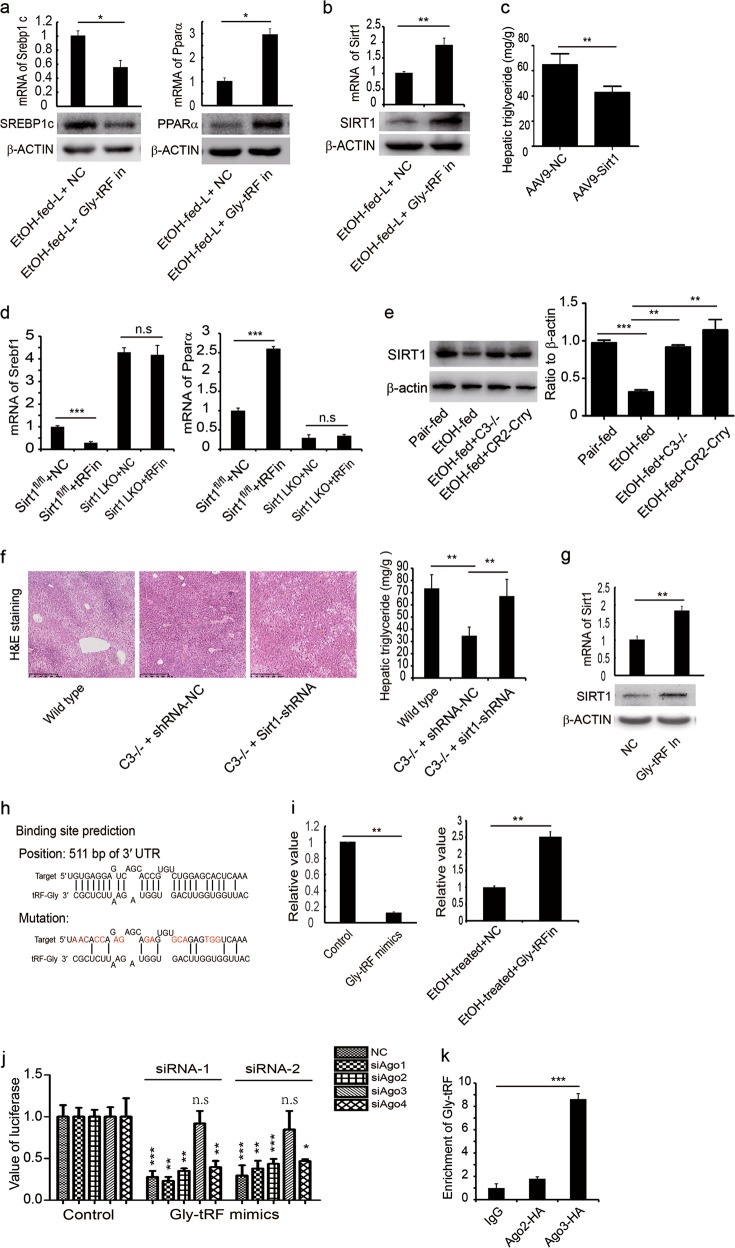
Figure 5 探究Gly-tRFs的下游分子
5a 代谢变化-关键酶
5b sirt1含量变化
5c sirt1AAV9敲除 表型检测
5d sirt1基因鼠 表型检测
5e 和C3联系起来 每个下游的分子都要和前面的关键分子联系起来
Hepatic tissues from mice treated with Gly-tRF inhibitors were subjected to transcriptome sequencing, and Gly-tRF was shown to be associated with lipid metabolism in AFLD mice 理由1 Gly-tRF inhibitors 后下游代谢发生很大变化
Previous studies have indicated that ethanol-induced attenuation of hepatic SIRT1 plays an important role in the pathogenesis of AFLD, and that stimulation of SIRT1 expression protected against the development of AFLD 理由2 SIRT1是控制下游代谢的关键转录因子(好的分子)
5h 之后都是找调控SIRT1的分子Ago3
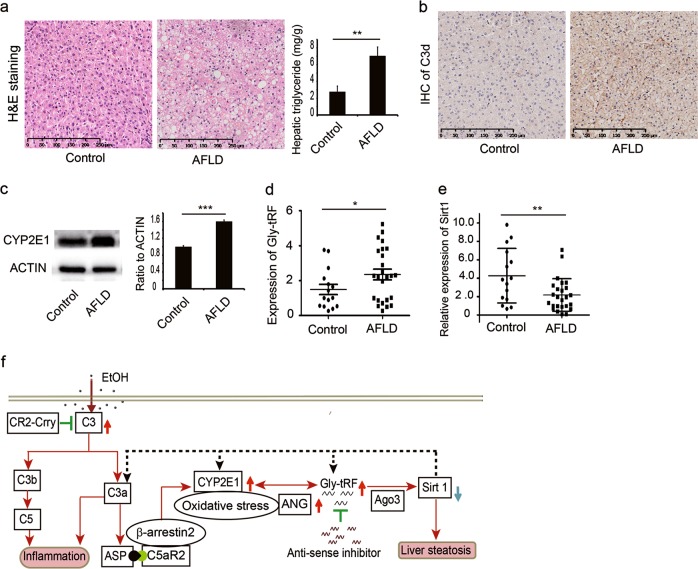
Figure 6 人的组织样本验证C3d, CYP2E1, Gly-tRF and Sirt1
C3转化酶的作用下, C3分子裂解成C3a和C3bC3b受血清中I因子H因子和蛋白酶的作用,又可逐级水解为iC3b,C3f,C3c,C3dg,C3d和C3g等片段,其中C3d由C3dg裂解而来。检测组织的话可以检测到这些游离态蛋白吗??
























 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








