google搜索gut bacteria psoriasis
#######################################################################
肠的结构:
根据【9】
大约8-9米长,所以指望饮食或者药物快速治愈银屑病是不可能的。
因为一口下去,能覆盖这个肠是不太可能的。
需要相当长的时间来治愈。
#######################################################################
肠道毒素类型
| 毒素类型 | 肠道细菌 | 毒素的化学形式 |
| 内毒素 | 革兰氏阴性菌[11] | LPS(脂多糖) |
| 外毒素 | 革兰氏阳性菌和少数革兰氏阴性菌[11] | 肠毒素、神经毒素、细胞毒素[10] |
【12】灭活乳酸菌可以去除食品中的黄曲霉毒素
#######################################################################
[1]中提到生酮饮食降低LPS水平,我们可以联想到之前网络中流行的观点:“生酮饮食治愈银屑病”,而LPS也与银屑病息息相关。
【2】中提到:
- ①大鼠食用高FODMAP饮食(HFM),可增加粪便中的革兰氏阴性菌,提高脂多糖(LPS)的浓度,引起肠道炎症、屏障功能障碍、内脏高敏感性(VH);
- ②抗生素处理可抑制上述症状,低FODMAP饮食(LFM)可逆转上述症状;
- ③给大鼠结肠内灌注LPS、肠易激综合征(IBS)患者或HFM大鼠的粪便上清,可造成肠道屏障功能障碍及VH,用LPS拮抗剂治疗或敲除TLR4可抑制病症;
- ④IBS患者的粪便LPS高于健康人,LFM处理4周显著改善IBS患者的症状并降低粪便LPS水平。
【3】
脂多糖(LPS)是革兰氏阴性细菌的主要成分,紧密结合到其细胞壁的最外层。因为其糖部分是水溶性的,而其脂质部分是脂溶性,所以LPS是两亲性物质,可溶于水和脂。
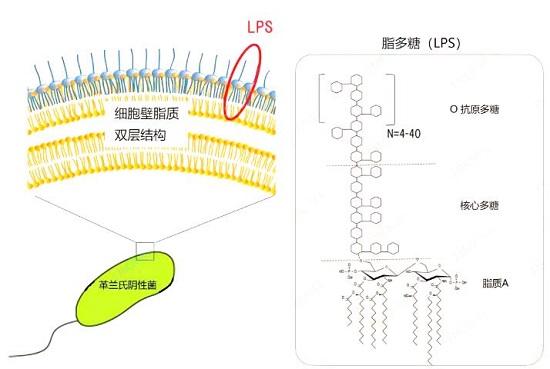
脂多糖的生物学作用
LPS在细菌正常生活状态时不释放,却通常在细菌菌体死亡破裂、人工方法裂解后或细胞活跃生长繁殖时会释放出来,其本身无毒性作用,但作为非特异性免疫原,当进入微循环后与宿主效应细胞(主要为单核细胞、巨噬细胞和中性粒细胞)相互作用分泌肿瘤坏死因子-α(TNF-α)、白细胞介素1(IL-1)、白细胞介素2(IL-2)、白细胞介素6(lL-6)、活性氧自由基(ROS)、一氧化碳(NO)等生物活性分子,引起机体发热、弥散性血管内凝血、多器官机能衰竭及休克等临床综合症。
LPS的毒性是通过LPS在细菌周围形成一层保护屏障以逃避抗生素的作用,作用于宿主细胞,产生炎性细胞因子,使机体内环境处于紊乱状态,引起内毒素血症、脓毒症等疾病。
LPS的活性
LPS具有广泛的生物学活性,不仅能激活T淋巴细胞、B淋巴细胞 、巨噬细胞 、自然杀伤细胞等免疫细胞,还能促进细胞因子生成,活化补体,对免疫系统发挥多方面的调节功能;LPS还具有免疫激活作用,能抗感染、抗辐射,增加机体免疫力,保护造血组织,并具有促白细胞增生的作用。
脂多糖水平升高的主要原因
酒精摄入量
营养不良
高脂饮食或高碳水化合物饮食
免疫细胞正在被激活(很可能是T细胞激活)
感染
肠漏
凝集素
暴饮暴食
社会隔离
吸烟
压力
##########################################################################
1) Fatigue
Fatigue is reliably caused in humans by the administration of LPS, as part of LPS-induced “sickness behavior” [1].(LPS会增加疲劳与炎症)
1) Fatigue
Fatigue is reliably caused in humans by the administration of LPS, as part of LPS-induced “sickness behavior” [1].(在一项针对39名参与者的试验中,注射LPS增加了社交脱节、抑郁和炎症(IL-6、TNF-a)的感觉[10]。
注射LPS后,动物海马中TNF-α的产生增加)
17) Cancer
Higher LPS levels were associated with an increased incidence of colorectal tumors in a study of 462 adults [38].(高水平的LPS更容易诱发癌症)
18) Alzheimer’s Disease
High levels of LPS and inflammatory cytokines were associated with Alzheimer’s disease in a study of 69 patients [40].(高水平的LPS与阿尔茨海默症相关)
22) Retinal Disease
Pigment cells of the retina died due to exposure to LPS-induced inflammatory cytokines (IL-6 and IL-8) [45].(高水平LPS容易导致视网膜色素细胞死亡)
1) Prebiotics
In three clinical trials of 119 obese and diabetic patients, inulin/oligofructose consumed daily for 8 to 12 weeks lowered LPS levels and inflammation, and increased bifidobacteria (beneficial gut bacteria) and blood sugar control [48, 49, 50].
Resistant starch lowered LPS, oxidative stress, and insulin resistance in another trial of 56 women with type 2 diabetes [51].
Inulin-like fructans increased Bifidobacterium levels, which was associated with lower LPS levels in a trial on 30 obese women [52].
2) Probiotics
In a trial on 30 patients with cirrhosis (liver damage), Lactobacillus GG taken for 8 weeks lowered LPS and TNF-alpha levels [53].
In another trial of 30 triathletes, daily supplementation of 30 billion CFU Lactobacillus and Bifidobacterium strains for 12 weeks reduced LPS pre-race and six days post-race [54].
In a trial on 44 HIV patients, 12-week treatment with Saccharomyces boulardii reduced LPS and systemic inflammation (IL-6) [55].
In a trial on 50 women given probiotics and a Japanese herbal medicine (Bofutsushosan), increased gut levels of the probiotic Bifidobacterium breve were linked to lower levels of LPS [56].
B. infantis 35624 reduced TNF-alpha and IL-6 production caused by LPS in a trial of 22 healthy participants [57].
Both pre- and probiotic supplementation has been linked with lower LPS levels, but larger trials are needed.
3) Polyphenols
Grape extract rich in polyphenols lowered blood LPS in a study of 29 adults [58].
In a study of 10 healthy people, consumption of a resveratrol and grape polyphenol drink suppressed the LPS, oxidative stress, and inflammatory stress response to a high-fat, high-carbohydrate meal [59].
In mice, a polyphenol-rich cranberry extract reduced LPS response to a high-fat meal [60].
4) Red Wine
Red wine consumption for 20 days increased Bifidobacterium and Prevotella bacteria levels, which were associated with reduced LPS levels in a study of 10 men [61].
Red wine polyphenols decreased LPS producing-bacteria and increased the number of fecal Bifidobacterium and Lactobacillus (gut barrier protectors) and butyrate-producing bacteria in a trial of 20 individuals [62].
Remember that the potential health effects of red wine may be outweighed by the multiple risks of alcohol, especially if consumed in high amounts.
5) Omega-3 Fatty Acids
High-dose omega-3 supplementation (3.6 g/day) reduced fever and moderately lowered inflammatory cytokines due to LPS administration in a trial of 60 healthy people [63].
Omega-3 supplementation (2.5 g/day) lowered LPS-stimulated IL-6 production and anxiety symptoms in another trial of 68 participants [64].
In mice, omega 3 supplementation and omega-6 reduction increased the production of alkaline phosphatase, caused favorable changes in gut bacteria composition, and lowered LPS production, gut permeability, and inflammation [65].
6) Olive Oil
A high-phenol olive oil breakfast limited the increases in LPS and inflammatory cytokines (NF-κB, IL-6, IL-1b, and CXCL1) in a clinical trial on 49 people with metabolic syndrome [66].
In a study of 28 healthy and obese people, consumption of a diet high in palmitic acid for 3 weeks increased blood TNF-a levels caused by LPS. However, consumption of a high-oleic acid (the main fat in olive oil) diet for 3 weeks lowered LPS-induced IL-1b, IL-18, IL-10, and TNF-a [67].
7) Orange Juice
When consumed with a high-fat meal, orange juice prevented the increase in LPS, oxidative stress, and inflammation compared to water or sugar water in a clinical trial on 30 people [68].
8) Peanuts
In a trial of 65 overweight men, consumption of a high-fat meal including peanuts lowered LPS levels compared to the same high-fat meal without peanuts. Consuming peanuts high in oleic acid had the strongest effect [69].
9) Bilberries
Consumption of 400 g/day of bilberries for 8 weeks reduced LPS, CRP, IL-6, and IL-12 in a small trial of 27 participants [70].
10) Exercise
Sedentary people have higher blood LPS levels than highly-trained people [71].
13) Lactoferrin(乳铁蛋白)
Lactoferrin is a protein found in milk, tears, and saliva that is part of the immune system and protects against bacteria and fungi.
Potential healthy ways to reduce LPS include cooking with olive oil and eating foods high in pre- and probiotics, polyphenols, and omega-3 fatty acids. Meditation and vagus nerve stimulation might also help, according to limited research data.(橄榄油烹饪,吃富含益生菌、多酚和ω-3脂肪酸的食物)
上面内容来自【4】
##########################################################################
下面内容来自【6】
LPS content both in cecal contents and blood was concomitantly increased by fat ingestion [9], and this increase of LPS was suppressed with oral administration of intestinal alkaline phosphatase, a LPS inactivating enzyme [9]. Oral administration of ampicillin and neomycin, broad-spectrum antibiotics that are poorly absorbed, also suppressed the increase in blood LPS concentration induced by a high-fat diet [10].These reports suggest that intestinal bacteria are an important source of LPS. In particular, Cani et al. demonstrated changes in intestinal flora (reduction in Bacteroides, Bifidobacterium, and Eubacterium) due to a high-fat diet. (脂肪摄入导致盲肠内容物和血液中LPS含量同时增加[9],口服LPS失活酶肠道碱性磷酸酶可抑制LPS含量的增加[9]。口服氨苄西林和新霉素(吸收不良的广谱抗生素)也能抑制高脂饮食引起的血LPS浓度升高[10]。这些报告表明,肠道细菌是LPS的重要来源。特别是,Cani等人证明了由于高脂肪饮食引起的肠道菌群变化(类杆菌、双歧杆菌和真杆菌减少)。因此,高脂饮食引起的肠道菌群失调作为代谢性内毒素血症的可能原因引起了人们的关注)
Changes in the intestinal bacteria due to ingestion of a high-fat diet have been studied in animals and humans and have been summarized in a review by Netto Candido et al. [11](已经在动物和人类中研究了因摄入高脂肪饮食引起的肠道细菌变化,并在Netto Candido等人的综述中进行了总结[11])
In animals, it has been reported that a high-fat diet increases the proportion of Firmicutes, Proteobacteria, and the ratio of Firmicutes to Bacteroidetes(另一方面,据报道,在人类中,高脂肪饮食摄入会增加类杆菌的比例,降低厚壁菌和变形菌的比例)
Devkota et al. evaluated the gut microbiota in C57BL/6 mice fed a low-fat diet, a high-fat diet with lard, or a high-fat diet with milk fat for 21 days [12]. In this experiment, both high-fat diets were isocaloric, rich in saturated fatty acids, and 37% of the ingested kcal were from fat. As a result, the proportion of Firmicutes increased and that of Bacteroidetes decreased in the gut microbiota of mice fed a high-fat diet containing lard, compared to mice fed a low-fat diet. In contrast, in mice fed a high-fat diet containing milk fat, the proportion of Firmicutes decreased and that of Bacteroidetes increased compared to the low-fat diet fed mice. Interestingly, Devkota et al. also identified specific bacteria that increased only by ingestion of a high-fat diet containing milk fat [12]. Compared to mice fed a low-fat diet, or a high-fat diet containing lard, mice fed with a high-fat diet containing milk fat had increased proportions of Bilophila wadsworthia, a sulfite-reducing bacterium, in gut microbiota. They also elucidated the mechanism underlying this increase; intake of milk fat increased the level of taurocholic acid in bile. Bilophila wadsworthia populations increased by utilizing sulfur components in taurocholic acid, causing intestinal inflammation in mice. An increase in total fecal bile acid and a concomitant increase in Bilophila wadsworthia in the gut microbiota was also reported in humans upon dietary intake of animal fat [13]. Natividad et al. also showed that increased Bilophila wadsworthia in mice fed a high-fat diet contributed to increased blood LPS levels (they measured soluble CD14 as a surrogate marker), increased fasting blood glucose levels, and the development of a fatty liver [14].
(Devkota等人评估了喂食低脂饮食、含猪油的高脂饮食或含乳脂的高脂饮食21天的C57BL/6小鼠的肠道微生物群[12]。在这个实验中,两种高脂肪饮食都是等热量的,富含饱和脂肪酸,37%的摄入的KCl来自脂肪。因此,与喂食低脂饮食的小鼠相比,喂食含猪油的高脂饮食的小鼠肠道微生物群中厚壁菌的比例增加,拟杆菌的比例减少。相反,与低脂饮食喂养的小鼠相比,在喂食含乳脂的高脂饮食的小鼠中,厚壁菌的比例减少,拟杆菌的比例增加。有趣的是,Devkota等人还发现了仅通过摄入含有乳脂的高脂肪饮食而增加的特定细菌[12]。与喂食低脂饮食或含猪油的高脂饮食的小鼠相比,喂食含乳脂的高脂饮食的小鼠肠道微生物群中嗜亚硫酸盐还原菌Bilophila wadsworthia的比例增加。他们还阐明了这种增长的机制;摄入乳脂会增加胆汁中牛磺胆酸的水平。通过利用牛磺胆酸中的硫成分,使Bilophila wadsworthia种群增加,导致小鼠肠道炎症。在人类饮食中摄入动物脂肪后,还报告了粪便总胆汁酸的增加以及肠道微生物群中嗜胆汁菌的伴随增加[13]。Natividad等人还表明,喂食高脂饮食的小鼠体内嗜Bilophila wadsworthia的增加有助于血液LPS水平的增加(他们测量可溶性CD14作为替代标记物)、空腹血糖水平的增加以及脂肪肝的发展[14]。)
It is also necessary to consider dietary LPS as a source of LPS. For example, milk has been reported to contain high concentrations of LPS in some commercial products [15]. Multiple animal studies have reported that ingested LPS may contribute to increased blood LPS levels. Specifically, Kaliannan et al. measured blood LPS levels 45 min after ingestion of LPS alone or corn oil and LPS in mice [9]. It showed that blood LPS levels were elevated when corn oil and LPS were co-administered. Lindenberg et al. reported that LPS concentrations in the blood were higher in mice fed a high-fat diet containing LPS than in mice fed a high-fat diet without LPS [16]. However, the effect of LPS levels in food on blood LPS levels has not been adequately studied in humans and further studies are needed.
(还需要考虑膳食LPS作为LPS的来源。例如,据报道,牛奶在一些商业产品中含有高浓度的LPS[15]。多项动物研究报告,摄入LPS可能导致血液LPS水平升高。特别是,Kaliannan等人在小鼠单独摄入LPS或玉米油和LPS 45分钟后测量了血LPS水平[9]。结果表明,当玉米油和LPS合用时,血液LPS水平升高。Lindenberg等人报告,喂食含LPS的高脂饮食的小鼠血液中的LPS浓度高于喂食不含LPS的高脂饮食的小鼠[16]。然而,食物中LPS水平对人类血液LPS水平的影响尚未得到充分研究,需要进一步研究)
The limulus amebocyte lysate assay used to measure LPS recognizes lipid A, a glycolipid moiety of LPS [17], but because lipid A is embedded in the outer membrane of gram-negative bacteria [18], elevated blood LPS levels suggest that LPS released from gram-negative bacteria is flowing into the blood. In an in vitro study with Escherichia coli, the concentration of free LPS in the culture medium increased with bacterial growth, but the addition of antibiotics stimulated further LPS release [19]. In addition, Jin et al. suggested that treatment with penicillin and erythromycin killed the gram-negative bacteria, Bacteroides and γ-Proteobacteria, leading to increased blood LPS levels in mice [20].
(青霉素、红霉素杀死革兰氏阴性菌会导致该细菌释放LPS进入血液,血液LPS水平升高)
Although the ratio of fat to energy intake varied, it has been reported that blood LPS levels increased by consumption of a high-fat diet with 30% of kcal ingested being from fat [32,33].Therefore, the reason for the lack of increase in blood LPS levels in the study of Reichardt et al. is not considered to be a difference in the fat content of the diet(高脂肪摄入会导致LPS升高)
It is reported that blood LPS is bound to various lipoproteins, with plasma LPS concentrations of 31%, 30%, 29%, and 10% for the very low-density lipoprotein (VLDL) fraction, low-density lipoprotein (LDL) fraction, high-density lipoprotein (HDL) fraction, and free LPS, respectively [36].
| 与血浆LPS结合的各类物质 | 比例 |
| 超低密度脂蛋白(VLDL) | 31% |
| 低密度脂蛋白(:LDL) | 30% |
| 高密度脂蛋白(HDL) | 29% |
| 游离LPS | 10% |
Increased LPS content has been reported in the livers of mice fed a high-fat diet [43], suggesting that the liver is an important site for LPS clearance. Ninety percent of the free LPS that entered the bloodstream is captured by liver resident macrophages (i.e., Kupffer cells) within 1 h [44]. LPS bound to HDL attaches primarily to sinusoidal epithelial cells of the liver [40,44], but it shows slower blood kinetics than free LPS, with 50% present in plasma even 1 h after administration and the amount accumulated in the liver accounted for only 15% of the dose [44]. LPS bound to HDL on the other hand is distributed widely to organs other than the liver, such as the kidney and adipose tissue [44]. LPS accumulated in the liver is inactivated by acyloxyacyl hydroxylase produced by Kupffer cells regardless of free or HDL-bound form [44]. Previously, in a mouse model of high-fat diet plus streptozotocin-induced non-alcoholic steatohepatitis-hepatocellular carcinoma, fecal LPS levels were continuously elevated from six weeks, while liver LPS levels were transiently elevated at eight weeks, followed by increased plasma LPS levels [45]. This report suggests that the liver acts as the first barrier against LPS entering from the intestinal tract and that liver dysfunction leads to elevated blood LPS levels. Interestingly, LPS administration in mice increased the expression of apolipoprotein AIV in the liver via TLR4, suggesting that the liver has a mechanism to increase HDL production and protect itself against LPS stimulation [46].(据报道,喂食高脂饮食的小鼠肝脏中LPS含量增加[43],表明肝脏是清除LPS的重要部位。进入血流的90%游离LPS在1小时内被驻留在肝脏的巨噬细胞(即枯否细胞)捕获[44]。与高密度脂蛋白结合的LPS主要附着于肝窦上皮细胞[40,44],但其血液动力学比游离LPS慢,即使给药后1小时,血浆中仍有50%的LPS存在,肝脏中的累积量仅占剂量的15%[44]。另一方面,与HDL结合的LPS广泛分布于肝脏以外的器官,如肾脏和脂肪组织[44]。累积在肝脏中的LPS被Kupffer细胞产生的酰氧基羟化酶灭活,无论游离形式或HDL结合形式如何[44]。此前,在高脂饮食加链脲佐菌素诱导的非酒精性脂肪性肝炎肝细胞癌的小鼠模型中,粪便LPS水平从6周开始持续升高,而肝脏LPS水平在8周时短暂升高,随后血浆LPS水平升高[45]。该报告表明,肝脏是阻止LPS从肠道进入的第一道屏障,肝功能障碍导致血LPS水平升高。有趣的是,给小鼠注射LPS可通过TLR4增加肝脏中载脂蛋白AIV的表达,这表明肝脏具有增加HDL生成和保护自身免受LPS刺激的机制[46])
Since metabolic endotoxemia has been implicated in gut dysbiosis, the effects of probiotics have been investigated. However, the results in humans are unfavorable (Table 1).(有张表格是吃哪些东西能让LPS下降)
In addition, Pei et al. studied whether low-fat yogurt could be administered before a meal to suppress the increase in blood LPS after a meal [50] and found no efficacy. On the other hand, there have been several reports of the efficacy of probiotics in animal studies (Table 3) [43,51,52,53,54,55]. Lactobacillus rhamnosus, Lactobacillus sakei, Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium longum, Bifidobacterium infantis, and Bacillus cereus are used as species, and the dosage ranges from 107 to 1010 CFU/day for four to twelve weeks. These animal studies used a high-fat diet, a high-fat high-sucrose diet, or a Zucker-Lepfa/fa obesity model. In addition to a significant decrease in blood LPS or LBP levels, improvement of obesity, glucose metabolism, and dyslipidemia was also observed. Since the effects of probiotics are strain-specific, it is expected that the effects of strains that have been effective in animal studies will be verified in humans.(此外,Pei等人研究了是否可以在饭前服用低脂酸奶来抑制饭后血LPS的增加[50],但没有发现任何效果。另一方面,在动物研究中已经有一些关于益生菌功效的报告(表3)[43,51,52,53,54,55]。鼠李糖乳杆菌、sakei乳杆菌、嗜酸乳杆菌、植物乳杆菌、长双歧杆菌、婴儿双歧杆菌和蜡样芽孢杆菌用作菌种,剂量范围为107至1010 CFU/天,持续4至12周。这些动物研究采用高脂肪饮食、高脂肪高蔗糖饮食或Zucker-Lepfa/fa肥胖模型。除了显著降低血LPS或LBP水平外,还观察到肥胖、糖代谢和血脂异常的改善。由于益生菌的作用是菌株特异性的,预计在动物研究中有效的菌株的作用将在人类身上得到验证。)
In intervention studies with oligofructose [57] and inulin [58,59], subjects with obesity, overweight subjects, and subjects with type 2 diabetes consumed 10–21 g of test substances for 8–12 weeks. Two of the three studies showed a significant decrease in blood LPS levels [57,58].(低聚果糖、菊糖)
In mice, chronic administration of LPS has been reported to induce hyperphagia by decreasing leptin sensitivity of afferent vagal nerves [66], and the reduced blood LPS levels and appetite suppression seen with galacto-oligosaccharide administration are of interest in supporting an association between LPS and appetite.(低聚半乳糖 降低LPS)
3.3. Polyphenols
Polyphenols are secondary metabolites found in plants and are responsible for protection against oxidative stress, UV damage, and pathogenic microorganisms [67]. Polyphenols are found in a wide range of foods, including vegetables, fruits, tea, beans, and spices, and their consumption has been reported to improve metabolic syndrome (decreased body weight, decreased blood pressure, improved glucose metabolism, and improved lipid metabolism) [68]. However, up to 27% of ingested polyphenols are detected in urine [69], suggesting that many of them are not absorbed and reach the large intestine [70]. Since polyphenols reaching the large intestine have been reported to alter the proportions of microbiota [71], it is expected that the effect of polyphenols against metabolic syndrome is mediated through the improvement of dysbiosis and of the accompanying metabolic endotoxemia. There are two human intervention studies investigating the relationship between polyphenol intake and blood LPS, both of which evaluated the inhibitory effect on postprandial elevation of blood LPS levels (Table 1) [72,73]. In the study performed by Ghanim et al., healthy individuals ingested capsules containing 100 mg of resveratrol and 75 mg of polyphenol 10 min before a 930-kcal high-fat, high-carbohydrate meal. Blood LBP levels up to 5 h after a meal were evaluated and showed increased blood LBP levels in the placebo group but not in the capsule group [72]. On the other hand, Clemente-Postigo et al. administered 272 mL of red wine to humans simultaneously with excessive fat and found no effect on either blood LPS or LBP levels [73]. The efficacy of polyphenols has been also reported in animal studies. The effects of grape seed proanthocyanin [29,33], resveratrol [74], apple-derived polymeric procyanidins [75], genistein [76], isoflavone [77], and syringarecinol [78] on blood LPS levels in animal models have been reported (Table 3). In particular, L’openz et al. reported that six-month administration of genistein to high-fat diet-fed mice reduced their blood LPS levels and improved their spatial memory ability [76]. Cho et al. administered syringalesinol to 40-week-old mice for 10 weeks and showed that the decrease in blood LBP levels was accompanied with suppression of changes in immune cells due to aging (decreased naive T cells and decreased T-cell proliferation) [78]. It has also been reported that adoption of a high-fat diet results in abnormal differentiation of bone marrow hematopoietic stem cells due to increased blood LPS levels [79], suggesting that the effect of syringalecinol on immunoaging might be also exerted in other models of metabolic endotoxemia.
(3.3. 多酚类物质
多酚是植物中的次生代谢物,负责保护植物免受氧化应激、紫外线损伤和病原微生物[67]。多酚广泛存在于各种食品中,包括蔬菜、水果、茶、豆类和香料,据报道,食用多酚可改善代谢综合征(体重下降、血压下降、糖代谢改善和脂质代谢改善)[68]。然而,在尿液中检测到多达27%的摄入多酚[69],这表明其中许多多酚没有被吸收并到达大肠[70]。由于有报道称到达大肠的多酚类物质会改变微生物群的比例[71],因此,预计多酚类物质对代谢综合征的作用是通过改善失调和伴随的代谢性内毒素血症来实现的。有两项人类干预研究调查了多酚摄入与血LPS之间的关系,这两项研究都评估了对餐后血LPS水平升高的抑制作用(表1)[72,73]。在Ghanim等人进行的研究中,健康人在930 kcal高脂肪、高碳水化合物膳食前10分钟摄入含有100 mg白藜芦醇和75 mg多酚的胶囊。对餐后5小时内的血LBP水平进行评估,发现安慰剂组的血LBP水平升高,而胶囊组的血LBP水平没有升高[72]。另一方面,Clemente Postigo等人同时给人服用了272毫升红葡萄酒和过量脂肪,并发现对血液LPS或LBP水平没有影响[73]。多酚类物质的功效在动物研究中也有报道。已经报道了葡萄籽原花青素[29,33]、白藜芦醇[74]、苹果衍生的聚合原花青素[75]、染料木素[76]、异黄酮[77]和丁香没食子酸[78]对动物模型血液LPS水平的影响(表3)。特别是,L'openz等人报告说,对高脂饮食喂养的小鼠给予染料木素6个月,可降低其血液LPS水平,并改善其空间记忆能力[76]。Cho等人给40周龄的小鼠服用丁香醛醇10周,结果表明,血液LBP水平的降低伴随着衰老导致的免疫细胞变化的抑制(原始T细胞减少,T细胞增殖减少)[78]。也有报道称,由于血液LPS水平升高,采用高脂饮食导致骨髓造血干细胞异常分化[79],这表明丁香醇对免疫老化的影响也可能在其他代谢性内毒素血症模型中发挥作用。)
3.4. Sulfated Polysaccharide
Sulfated polysaccharides are widely present in animal tissues and seaweed and are used industrially as anticoagulants, pharmaceuticals, and gelling agents for foods. The effect of sulfated polysaccharides on metabolic endotoxemia has been studied only in animals (Table 3). Intervention studies with sea cucumber-derived sulfated polysaccharides [80,81], acaudina molpadioides-derived fucosylated chondroitin sulfate [82], chicken-derived chondroitin sulfate [83] or fucoidan [84] have been performed. Of these studies, two showed that administration of sulfated polysaccharides to high-fat diet-fed mice increased the amount of short-chain fatty acids in the intestinal tract, decreased the blood LPS or LBP concentration and attenuated weight gain [80,82]. Zhu et al. also reported the same effect of sulfated polysaccharides in chow-fed lean mice [81]. Liu et al. demonstrated that exhaustive exercise with a treadmill significantly impaired kidney function, decreased fecal butyrate levels, changed intestinal morphology, and induced metabolic endotoxemia [83]. Their study is interesting in showing that exercise stress also increased blood LPS levels, and that dietary factors are also effective in the model mice.(3.4. 硫酸多糖
硫酸多糖广泛存在于动物组织和海藻中,在工业上用作抗凝剂、药物和食品胶凝剂。硫酸多糖对代谢性内毒素血症的影响仅在动物中进行了研究(表3)。已经对海参衍生的硫酸多糖[80,81]、acaudina molpadioides衍生的岩藻糖基硫酸软骨素[82]、鸡肉衍生的硫酸软骨素[83]或岩藻糖胶[84]进行了干预研究。其中两项研究表明,向高脂饮食喂养的小鼠施用硫酸多糖可增加肠道内短链脂肪酸的数量,降低血液LPS或LBP浓度,并减少体重增加[80,82]。Zhu等人还报告了硫酸多糖对周饲瘦肉小鼠的相同作用[81]。Liu等人证明,在跑步机上进行力竭运动会显著损害肾功能,降低粪便丁酸水平,改变肠道形态,并诱发代谢性内毒素血症[83]。他们的研究有趣地表明,运动应激也会增加血LPS水平,饮食因素对模型小鼠也有效。)
3.5. Other Dietary Components/Extracts/Foods
In the study by Abboud et al., obese or over weight subjects ingested 30 g of glutamine per day for eight weeks (Table 1) [85]. As a result, their blood LPS levels and waist circumference decreased. In an epidemiological study conducted with healthy subjects, 25-hydroxy vitamin D was reported to negatively correlate to blood LPS levels (Table 2) [86]. The protective effect of vitamin D is supported by animal studies in which vitamin D-deficient mice, exposed to a bacterial pathogen, exhibited lower LPS detoxification activity of the intestine and greater endotoxin translocation [87]. The effect of other dietary components, including tetrahydro iso-alpha acid [88], rhein [89], phlorizin [90], capsaicin [91], rutin [92], and lycopene [93] on blood LPS levels in animals has also been reported (Table 3). Among them, administration of tetrahydro iso-alpha acid [88], phlorizin [90], or rutin [92] to high-fat diet-fed mice or db/db mice improved metabolic impairment. Administration of rhein [89], or lycopene [93] to high-fat diet-fed mice showed a unique effect; they not only reduced blood LPS levels but also prevented high-fat diet-induced memory impairment. Kang et al. showed that administration of antibiotics to mice given capsaicin abolished the effect of capsaicin on blood LPS levels [91]. They also showed that capsaicin-induced protection against high-fat diet-induced blood LPS increase is transferrable by fecal microbiota transplantation.
It has also been reported that intervention with crude food extracts or the food itself can lower blood LPS levels in animals (Table 3). We studied the effect of broccoli sprout extract, enriched in functional glucosinolate “glucoraphanin” (details are described in Section 4) [94]. Anhê et al. examined the effects of extracts from cranberry [95] or camu camu [96]. Camu camu is an Amazonian fruit that contains an abundance of vitamin C and flavonoids such as ellagic acid, ellagitannins, and proanthocyanidins. Administration of camu camu extracts to high-fat/high-sucrose diet-fed mice reduced plasma bile acid pool size, altered gut microbiota composition, and reduced blood LPS levels. Dey et al. reported that administration of green tea extract to high-fat diet-fed mice suppressed inflammation and gut permeability especially in the ileum and colon, and reduced LPS influx from the portal vein [34]. The reduction of blood LPS levels by feeding with Tartary buckwheat protein was reported by Zhou et al. [32]. This study is valuable in that it elucidates one of the underlying mechanisms by which plant protein intake leads to improvement of metabolic abnormalities. Intervention studies with cocoa [97], nopal [98], and steamed fish meat [99] have been performed. Among these, Zhang et al. performed unique experiments [99]. They divided mice into four groups, and fed them ad libitum with normal chow, steamed fish, pork or beef at 9:00 and 18:00 daily for eight weeks. As a result, only mice group fed with steamed fish showed decreased blood LBP levels compared to the other three groups.
(3.5. 其他膳食成分/提取物/食物
在Abboud等人的研究中,肥胖或超重受试者连续八周每天摄入30 g谷氨酰胺(表1)[85]。结果,他们的血LPS水平和腰围下降。在对健康受试者进行的流行病学研究中,25-羟基维生素D与血液LPS水平呈负相关(表2)[86]。维生素D的保护作用得到了动物研究的支持,在动物研究中,维生素D缺乏的小鼠暴露于细菌病原体,表现出较低的肠道LPS解毒活性和较大的内毒素移位[87]。其他饮食成分,包括四氢异α酸[88]、大黄酸[89]、根皮苷[90]、辣椒素[91]、芦丁[92]和番茄红素[93]对动物血液LPS水平的影响也有报道(表3)。其中,向高脂饮食喂养的小鼠或db/db小鼠施用四氢异α酸[88]、根皮苷[90]或芦丁[92]可改善代谢损伤。给高脂饮食喂养的小鼠服用大黄酸[89]或番茄红素[93]显示出独特的效果;他们不仅降低了血液LPS水平,还防止了高脂肪饮食引起的记忆障碍。Kang等人证明,给给予辣椒素的小鼠服用抗生素可消除辣椒素对血液LPS水平的影响[91]。他们还表明,辣椒素诱导的对高脂饮食诱导的血LPS增加的保护作用可通过粪便微生物群移植进行转移。
也有报道称,用粗食物提取物或食物本身进行干预可以降低动物的血LPS水平(表3)。我们研究了西兰花芽提取物的作用,该提取物富含功能性硫代葡萄糖苷“葡萄糖拉菲”(详情见第4节)[94]。Anhê等人研究了蔓越莓[95]或卡姆卡姆[96]提取物的作用。Camu Camu是一种亚马逊水果,含有丰富的维生素C和类黄酮,如鞣花酸、鞣花鞣花素和原花青素。向高脂/高糖饮食喂养的小鼠施用camu-camu提取物可减少血浆胆汁酸池大小,改变肠道微生物群组成,并降低血LPS水平。Dey等人报告称,向高脂饮食喂养的小鼠施用绿茶提取物可抑制炎症和肠道通透性,尤其是回肠和结肠,并减少门静脉LPS流入[34]。Zhou等人[32]报告了通过喂食苦荞蛋白降低血液LPS水平的情况。这项研究很有价值,因为它阐明了植物蛋白质摄入导致代谢异常改善的潜在机制之一。对可可[97]、nopal[98]和蒸鱼肉[99]进行了干预研究。其中,Zhang等人进行了独特的实验[99]。他们将老鼠分为四组,每天9:00和18:00随意喂食正常食物、蒸鱼、猪肉或牛肉,持续八周。因此,与其他三组相比,只有喂食清蒸鱼的小鼠组血液LBP水平降低。)
3.6. Chinese Medicines
The effect of the Chinese medicines; geniposide + chlorogenic acid [100], potentilla discolor bunge water extract [101], ganoderma lucidum mycelium water extract [102], semen hoveniae extract [103], and shenling baizhu powder [104] on blood LPS levels have been reported in animals (Table 3). The combination of geniposide and chlorogenic acid is included in a traditional Chinese medicine, Qushi Huayu Decoction. Peng et al. indicated that administration of geniposide and chlorogenic acid to high-fat diet-fed mice restored colonic tight junctions by inhibiting down-regulation of RhoA/Rho-associated kinase signaling, and reduced blood LPS levels and hepatic LBP protein levels [100]. Han et al. examined the effect of potentilla discolor bunge water extract in type 2 diabetic mice induced by high-fat diet feeding and streptozotocin injection [101]. The results showed that fecal LPS levels in the type 2 diabetic model mice were significantly increased compared to the control normal mice. The administration of potentilla discolor bunge water extract to mice reduced fecal LPS levels, decreased blood LPS levels and increased the expression levels of tight junction proteins (Claudin-3, ZO-1, and Occludin) in the colon. Chang et al. studied the effect of ganoderma lucidum mycelium, a Basidiomycete fungus [102]. They showed the dose-dependent effect of ganoderma lucidum mycelium water extract on blood LPS reduction, suggesting that high molecular weight polysaccharides (>300 kDa) isolated from the extract is an effective component. Ping et al. reported that the extract of semen hoveniae, a seed of Hovenia dulcis Thunb rich in dihydromyricetin and quercetin, decreased blood LPS levels in a mouse model of alcohol-induced liver injury [103]. It has been reported that administration of shenling baizhu, a mixture of ten different traditional Chinese medicinal herbs, to high-fat diet-fed mice decreased LPS levels in the portal vein [104].(3.6. 中药
中药的作用;已报告geniposide+绿原酸[100]、翻白草水提取物[101]、灵芝菌丝体水提取物[102]、枳实提取物[103]和神灵白术粉[104]对动物血液LPS水平的影响(表3)。栀子苷和绿原酸的组合包含在传统中药祛湿化瘀汤中。Peng等人指出,通过抑制RhoA/Rho相关激酶信号的下调,并降低血液LPS水平和肝脏LBP蛋白水平,向高脂饮食喂养的小鼠施用京尼平苷和绿原酸可恢复结肠紧密连接[100]。Han等人研究了翻白草水提取物对高脂饮食喂养和链脲佐菌素注射液诱导的2型糖尿病小鼠的影响[101]。结果表明,与对照正常小鼠相比,2型糖尿病模型小鼠的粪便LPS水平显著升高。向小鼠施用翻白草水提取物可降低粪便LPS水平,降低血液LPS水平,并增加结肠中紧密连接蛋白(Claudin-3、ZO-1和Occludin)的表达水平。Chang等人研究了担子菌灵芝菌丝体的作用[102]。他们显示了灵芝菌丝体水提取物对降低血LPS的剂量依赖效应,表明从提取物中分离的高分子量多糖(>300kDa)是一种有效成分。Ping等人报道,在酒精诱导的肝损伤小鼠模型中,富含二氢杨梅素和槲皮素的枳实种子枳实提取物降低了血液LPS水平[103]。据报道,将十种不同中草药的混合物神灵白术用于高脂饮食喂养的小鼠,可降低门静脉的LPS水平[104]。)
3.7. Dietary Habits
In relation to dietary habits, Kopf et al. conducted an intervention study in humans with BMI > 25 kg/m2 and low intake of whole grains, fruits, and vegetables (Table 1) [65]. During the weekly interview, the subjects themselves selected the vegetables and fruits to be eaten the following week from apples, bananas, blueberries, clementines, grapes, pears, strawberries, broccoli, carrots, cauliflower, celery, green beans, green leaf lettuce, peas, spinach, sweet pepper, and tomatoes. The subjects ate these fruits and vegetables for 21 to 30 servings/week (at least three servings/day) for six weeks. As a result, compared to control group in that dietary habits were not or minimal changed, average daily intake of refined grains was 1/3, fruit intake was doubled, and vegetable intake was four times, leading to a significant reduction in blood LBP levels and IL-6 levels. An epidemiological study by Ahola et al. in patients with type 1 diabetes has shown a negative correlation between several dietary patterns and blood LPS levels: These dietary patterns are “Fish” (frequently eats fish dishes), “Healthy snack” (frequently eats fruits, berries, fresh vegetables, yoghurt, low-fat cheese, and does not drink many soft drinks) and “Modern” (frequently eats poultry, pasta, rice, meat dishes, fried and grilled foods, and fresh vegetables) (Table 2) [105]. In the epidemiological study by Ahola et al., no significant correlation was found between blood LPS levels and intake of energy, carbohydrates, fats, proteins, or dietary fiber. In regard to the absence of a significant positive correlation between blood LPS levels and fat intake (the believed cause of blood LPS elevation in humans and animals), the authors consider that the previously reported amount or proportion of fat intake may be greater than the intake in the normal diet. Similarly, Amar et al. reported no significant correlation between fat intake and blood LPS levels in 201 subjects [106]. In the same study, Amar et al. reported a positive correlation between total energy intake and blood LPS levels [106]. The effect of caloric restriction on blood LPS levels have been reported in both humans and mice. Ott et al. reported that, in women with a BMI of 30 kg/m2 or more, intake of a defined formula diet of 800 kcal/day for four weeks decreased blood LBP levels, and following intake of the normal diet (1800 kcal/day), blood LBP levels returned to the initial levels (Table 1) [107]. Even in mice, caloric restriction of 30% [108] or 40% [109] has been reported to decrease blood LPS or LBP levels (Table 3). A common finding in these reports in mice is that blood LPS or LBP levels are reduced by calorie restriction compared to ad libitum even in normal chow-fed mice. This suggests that the influx of LPS into the bloodstream is not limited to the specific conditions of excessive fat intake but can also occur by some mechanism in the normal diet.(3.7. 饮食习惯
关于饮食习惯,Kopf等人对体重指数>25 kg/m2且全谷物、水果和蔬菜摄入量较低的人进行了干预研究(表1)[65]。在每周访谈中,受试者自己从苹果、香蕉、蓝莓、克莱门汀、葡萄、梨、草莓、花椰菜、胡萝卜、花椰菜、芹菜、青豆、绿叶莴苣、豌豆、菠菜、甜椒和西红柿中选择下周要吃的蔬菜和水果。受试者每周食用这些水果和蔬菜21至30份(每天至少3份),持续6周。因此,与对照组相比,饮食习惯没有改变或变化很小,精制谷物的平均每日摄入量为1/3,水果摄入量增加了一倍,蔬菜摄入量增加了四倍,从而显著降低了血液LBP水平和IL-6水平。Ahola等人对1型糖尿病患者进行的一项流行病学研究表明,几种饮食模式与血LPS水平呈负相关:这些饮食模式是“鱼”(经常吃鱼菜),“健康零食”(经常吃水果、浆果、新鲜蔬菜、酸奶、低脂奶酪,不喝很多软饮料)和“现代”(经常吃家禽、面食、米饭、肉类菜肴、油炸和烧烤食品以及新鲜蔬菜)(表2)[105]在Ahola等人的流行病学研究中,未发现血LPS水平与能量、碳水化合物、脂肪、蛋白质或膳食纤维摄入之间存在显著相关性。关于血LPS水平与脂肪摄入之间缺乏显著正相关性(据信是人和动物血LPS升高的原因)作者认为,先前报道的脂肪摄入量的数量或比例可能大于正常饮食中的摄入量。同样,阿马尔等人报告201名受试者的脂肪摄入量与血液LPS水平没有显著相关性(106)。在同一研究中,Amar等人报告了总能量摄入与血液LPS水平之间的正相关[106]。已报告热量限制对人类和小鼠血液LPS水平的影响。Ott等人报告,在BMI为30 kg/m2或以上的女性中,连续四周摄入800 kcal/天的规定配方饮食可降低血液LBP水平,并且在摄入正常饮食(1800 kcal/天)后,血液LBP水平恢复到初始水平(表1)[107]。即使在小鼠中,据报道限制30%[108]或40%[109]的热量也会降低血液LPS或LBP水平(表3)。在这些研究报告中,小鼠的一个常见发现是,即使在正常的周粮喂养的小鼠中,与随意进食相比,热量限制也会降低血LPS或LBP水平。这表明,LPS流入血流不仅限于脂肪摄入过多的特定情况,而且在正常饮食中也可能通过某种机制发生
)
4. Association of Dietary Factor-Induced Reduction of Blood LPS and Modulation of Gut Microbiota
Although few studies have evaluated the relationship between the effect of dietary factors on blood LPS and intestinal flora in humans, several studies have evaluated intestinal flora in oligosaccharide intervention studies (Table 1). A common finding in these reports is an increase in Bifidobacterium. Bifidobacterium has been reported to enhance the intestinal tight junction by preserving claudin 4 and occludin localization at tight junctions, and inhibit permeability in mice with colitis [115]. Similarly, in human colonic epithelial cell line T84, the addition of culture supernatant of Bifidobacterium has been reported to enhance barrier function through increased expression of tight junction protein, suggesting that some humoral factors contribute to improved intestinal barrier function [116]. Increased expression of tight junction protein in Bifidobacterium-treated mice has been reported to be associated with increased short-chain fatty acids (acetic acid, butyric acid, and propionic acid) in the intestinal tract [117]. These short-chain fatty acids have been reported in the human colonic epithelial cell line caco-2 to act as an energy source for epithelial cells to protect themselves, and also act as a histone deacetylase inhibitor which inhibit Nod-like receptor P3 inflammasomes to maintain the barrier function of epithelial cells [118]. These results suggest that the increase in Bifidobacterium induced by oligosaccharide intake decreases blood LPS levels through the improvement of the barrier function of the intestinal tract. In addition, dietary factors that increase Bifidobacterium are expected to reduce blood LPS levels.(4.饮食因素诱导的血LPS降低与肠道微生物群调节的关系
虽然很少有研究评估了饮食因素对人体血液LPS和肠道菌群的影响之间的关系,但有几项研究在低聚糖干预研究中评估了肠道菌群(表1)。这些报告中的一个常见发现是双歧杆菌增多。据报道,双歧杆菌通过保留紧密连接处的claudin 4和occludin定位来增强肠道紧密连接,并抑制结肠炎小鼠的通透性[115]。类似地,在人类结肠上皮细胞系T84中,已报道添加双歧杆菌培养上清液通过增加紧密连接蛋白的表达来增强屏障功能,这表明一些体液因素有助于改善肠屏障功能[116]。据报道,双歧杆菌治疗的小鼠紧密连接蛋白的表达增加与肠道内短链脂肪酸(乙酸、丁酸和丙酸)的增加有关[117]。据报道,这些短链脂肪酸存在于人类结肠上皮细胞系caco-2中,可作为上皮细胞保护自身的能量来源,也可作为组蛋白去乙酰化酶抑制剂,抑制Nod样受体P3炎性体以维持上皮细胞的屏障功能[118]。这些结果表明,通过改善肠道屏障功能,低聚糖摄入诱导的双歧杆菌增加降低了血LPS水平。此外,增加双歧杆菌的饮食因素有望降低血液LPS水平。
)
Lactobacillus, Bacteroides, Akkermansia, Roseburia, and Prevotella are possible bacterial genera that may contribute to the reduction of blood LPS levels. Lactobacillus is a gram-positive bacterium that produces large amounts of lactic acid during carbohydrate fermentation. The probiotic contribution of Lactobacillus to the regulation of metabolic endotoxemia is studied (Table 3). Administration of Lactobacillus rhamnosus CNCM I-4036 to obese Zucker-Leprfa/fa rats decreased the mRNA expression levels of endothelin receptor type B (Ednrb) in the intestinal mucosa, and reduced the blood LBP level [52]. Reduction of Ednrb decreases the density of negative charge of the colonic mucin layer, leading to an increase in the ability of the mucin layer to adsorb microparticles and bacteria, thereby inhibiting their penetration through the colonic mucosa [119]. Lactobacillus sakei OK67 and PK16 are reported to suppress high-fat diet-induced colitis, and to reduce the fecal Proteobacteria population and fecal LPS levels in mice [53]. In addition to the previous reports described in Table 3, it has been reported that oral administration of Lactobacillus reuteri ZJ617 suppresses LPS-induced apoptosis of intestinal epithelial cells and maintains the intestinal barrier function [120]. We have described in Section 2.2 that LPS is absorbed from the intestinal tract during lipid absorption. Interestingly, oral ingestion of Lactobacillus acidophilus ATCC 4356 in mice has been reported to reduce the mRNA levels of Niemann-Pick C1-like 1, which is involved in lipid absorption in the intestine, and in the suppression of cholesterol absorption [121]. Taken together, this suggests that Lactobacillus contributes to a decrease in blood LPS levels through strengthening the intestinal barrier, reducing the amount of LPS in feces, and suppressing lipid absorption. As described in Table 4, Bifidobacterium, oligofructose, galacto-oligosaccharide, syringaresinol, acaudina molpadioides-derived fucosylated chondroitin sulfate, green tea extract, Tartary buckwheat protein, nopal, semen hoveniae extract, and 30% caloric restriction are dietary factors that increase the proportion of Lactobacillus in the gut microbiota. Among them, the amylolytic Bifidobacterium strain is reported to stimulate the growth of a nonamylolytic Lactobacillus probably by producing intermediate metabolites of starch metabolism [122]. Oligosaccharides (oligofructose and galacto-oligosaccharide) were reported to support the growth of Lactobacillus as prebiotics [123]. Green tea extract [124] and buckwheat-resistant starch [125] were reported to promote the growth of Lactobacillus in a fermentation assay. On the other hand, in an in vitro fermentation assay using gut microbiota, it was reported that fucosylated chondroitin sulfate promotes the growth of Bacteroides, Bifidobacterium, and Clostridium, while the number of Lactobacillus decreases [126]. Thus, the mechanism by which Lactobacillus increased in mice fed with fucosylated chondroitin sulfate needs to be further studied. The mechanism by which the proportion of Lactobacillus in gut microbiota increases due to calorie restriction also remains unknown. As it has been reported that the bacteria adapted to the nutritional environment can grow predominantly in the gut microbiota consortium [127], Lactobacillus might be able to grow even under malnutrition. The effect of syringaresinol, nolpal, and semen hoveniae on the growth of Lactobacillus has not been revealed.
Bacteroides is a gram-negative obligate anaerobe. Hooper et al. reported that Bacteroides thetaiotaomicron, a prominent component of the normal mouse and human intestinal microflora, modulates expression of genes involved in mucosal barrier fortification [128]. The administration of Bacteroides fragilis HCK-B3 and Bacteroides ovatus ELH-B2 to mice attenuated LPS-induced intestinal inflammation, by either modulating cytokine production or restoring the Treg/Th-17 balance [129]. On the other hand, in a state in which no dietary fiber is ingested, it has been suggested that Bacteroides degrades the mucin layer of the intestinal tract, decreases the barrier function of mucus, and induces inflammation [130]. Therefore, it should be noted that depending on the diet of the host, Bacteroides can act as either probiotics or pathobionts. As described in Table 4, an increase in Bacteroides was reported in four out of five intervention studies with sulfated polysaccharides. Bacteroides is a unique bacterium among gut flora that has degrading enzymes corresponding to various sulfated polysaccharides [131] and is able to utilize sulfated polysaccharides such as heparin [131], heparan sulfate [131], and chondroitin sulfate [132] as energy sources. It is therefore thought that intake of sulfate polysaccharide preferentially nourishes Bacteroides in gut flora and suppresses metabolic endotoxemia via its anti-inflammatory and barrier function-enhancing effects.
(
乳酸杆菌、类杆菌、阿克曼菌、玫瑰杆菌和普氏杆菌可能是有助于降低血液LPS水平的细菌属。乳酸杆菌是一种革兰氏阳性细菌,在碳水化合物发酵过程中产生大量乳酸。研究了乳酸杆菌对调节代谢性内毒素血症的益生菌作用(表3)。向肥胖Zucker-Leprfa/fa大鼠施用鼠李糖乳杆菌CNCM I-4036可降低肠粘膜中内皮素B型受体(Ednrb)的mRNA表达水平,并降低血LBP水平[52]。Ednrb的减少降低了结肠粘蛋白层的负电荷密度,导致粘蛋白层吸附微粒和细菌的能力增加,从而抑制其穿透结肠粘膜[119]。据报道,sakei OK67乳酸杆菌和PK16可抑制高脂饮食诱导的结肠炎,并可减少小鼠粪便中的变形菌群和粪便LPS水平[53]。除表3中所述的先前报告外,据报道,口服reuteri乳酸杆菌ZJ617可抑制LPS诱导的肠上皮细胞凋亡,并维持肠屏障功能[120]。我们在第2.2节中描述了脂多糖在脂质吸收过程中从肠道吸收。有趣的是,据报道,小鼠口服嗜酸乳杆菌ATCC 4356可降低参与肠道脂质吸收和抑制胆固醇吸收的Niemann-Pick C1样1的mRNA水平[121]。综上所述,这表明乳酸杆菌通过加强肠道屏障、减少粪便中LPS的数量和抑制脂质吸收,有助于降低血LPS水平。如表4所述,双歧杆菌、低聚果糖、低聚半乳糖、丁香树脂醇、acaudina molpadioides衍生岩藻糖基化硫酸软骨素、绿茶提取物、苦荞蛋白、nopal、枳实提取物、,30%的热量限制是增加肠道微生物群中乳酸杆菌比例的饮食因素。其中,据报道,淀粉分解双歧杆菌菌株可能通过产生淀粉代谢的中间代谢物来刺激非淀粉分解乳酸杆菌的生长[122]。据报道,低聚糖(低聚果糖和低聚半乳糖)作为益生元支持乳酸杆菌的生长[123]。据报道,绿茶提取物[124]和荞麦抗性淀粉[125]在发酵试验中可促进乳酸杆菌的生长。另一方面,在使用肠道微生物群进行的体外发酵试验中,据报道岩藻糖基硫酸软骨素促进类杆菌、双歧杆菌和梭菌的生长,而乳酸杆菌的数量减少[126]。因此,食用岩藻糖基化硫酸软骨素的小鼠中乳酸杆菌增加的机制需要进一步研究。由于热量限制,肠道微生物群中乳酸杆菌比例增加的机制也尚不清楚。据报道,适应营养环境的细菌主要在肠道微生物群中生长[127],即使在营养不良的情况下,乳酸杆菌也可能生长。丁香树脂醇、诺尔帕尔和枳实对乳酸杆菌生长的影响尚未揭示。
类杆菌是一种革兰氏阴性专性厌氧菌。Hooper等人报道,类杆菌thetaiotaomicron是正常小鼠和人类肠道微生物区系的重要组成部分,可调节参与粘膜屏障强化的基因表达[128]。向小鼠施用脆弱类杆菌HCK-B3和卵形类杆菌ELH-B2可通过调节细胞因子的产生或恢复Treg/Th-17平衡来减轻LPS诱导的肠道炎症[129]。另一方面,在不摄入膳食纤维的状态下,有人认为类杆菌会降解肠道的粘蛋白层,降低粘液的屏障功能,并诱发炎症[130]。因此,应注意,根据宿主的饮食,类杆菌可作为益生菌或致病菌。如表4所述,五分之四的硫酸多糖干预研究报告类杆菌增加。类杆菌是肠道菌群中的一种独特细菌,具有与各种硫酸多糖相对应的降解酶[131],能够利用硫酸多糖,如肝素[131]、硫酸乙酰肝素[131]和硫酸软骨素[132]作为能源。因此,人们认为摄入硫酸多糖优先营养肠道菌群中的类杆菌,并通过其抗炎和屏障功能增强作用抑制代谢性内毒素血症。)
Akkermansia is a mucin-adherent intestinal bacterium [133], which grows by degrading mucin [134], and produces propionic acid, a short-chain fatty acid [135]. In addition, Akkermansia promotes butyrate production, by supporting the growth of Anaerostipes caccae through mucin degradation [136]. As noted above, these short-chain fatty acids are known to enhance intestinal barrier function. In addition, it has been reported that Akkermansia-derived extracellular vesicles administered in mice are localized to the large intestine, and directly enhance intestinal barrier function by increasing epithelial cell expression of tight junction proteins [137]. Furthermore, oral administration of Akkermansia to mice inhibited high-fat diet-induced thinning of the mucin layer, reduced blood LPS concentration, and inhibited obesity and abnormal glucose metabolism [138]. Akkermansia has been reported to be negatively correlated with obesity (waist-to-hip ratio and subcutaneous adipocyte diameter) and diabetes mellitus (glucose intolerance states), and is attracting attention as a next-generation probiotic [139]. Among the dietary factors that increase the proportion of Akkermansia in the gut flora, polyphenols are intriguing because most of intervention studies with polyphenols (apple-derived polymeric procyanidins, genistein, and isoflavone) or polyphenol-rich food extracts (camu camu extract, cranberry extract, and green tea extract) consistently reported an increase of Akkermansia (Table 4). Anhê et al. reported that cranberry extract administration to mice increased colonic Kruppel-like factor 4 (a marker of goblet cells) and Muc2 mRNA expression, suggesting that polyphenols enhance mucin production and support the growth of Akkermansia [95]. On the other hand, direct prebiotic action of polyphenols to Akkermansia has been reported in a study using the Simulator of Human Intestinal Microbial Ecosystem (SHIME®) [140].
Roseburia [141] is an enteric bacterium that utilizes dietary fiber and may enhance intestinal barriers by producing butyric acid. It has been reported that administration of Roseburia to mice enhanced differentiation of regulatory T cells in the intestinal lamina propria and suppressed intestinal inflammation [142]. As described in Table 4, oligofructose, apple-derived polymeric procyanidins, sea cucumber-derived sulfated polysaccharide, camu camu extract, and ganoderma lucidum mycelium water extract were reported to increase the proportion of Roseburia in the gut flora. Roseburia metabolizes oligofructose into fructose, which is used for growth, but for this process, acetic acid that is produced by Bifidobacterium is required [143]. Therefore, in order to grow Roseburia by oligofructose intake, it is necessary to pay attention to the symbiotic relationship with other intestinal bacteria and the amount of short-chain fatty acids in the intestine. Other dietary factors, procyanidins, sea cucumber-derived sulfated polysaccharide, camu camu extract, and ganoderma lucidum mycelium, have not been studied for their prebiotic function for Roseburia.
It has been suggested that LPS from Prevotella has fewer phosphate and acyl moieties contributing to endotoxin activity, resulting in a lower TLR4 stimulatory capacity than LPS from Salmonella [144]. Therefore, by increasing the population of Prevotella in the intestinal flora, endotoxin activity in the intestinal contents and damage to intestinal epithelial cells might be decreased, leading to the reduction of blood LPS levels. On the other hand, Prevotella produces succinate as a metabolite of sugar metabolism [145]. It has also been reported that succinate from intestinal bacteria is utilized by and promotes growth of Salmonella serovar Typhimurium [146] and Clostridium difficile [147], which are the pathogens of pseudomembranous colitis. Succinate has also been reported to induce colitis via succinate receptors and to promote colonic fibrosis [148]. In addition, proportion of Prevotella in the gut flora has been reported to be positively correlated with blood LPS levels in patients with type 2 diabetes [149]. Thus, an increase in the proportion of Prevotella does not necessarily have a positive effect on intestinal health. It is necessary to carefully investigate the contribution of Prevotella to blood LPS levels.(
Akkermansia是一种粘蛋白粘附的肠道细菌[133],通过降解粘蛋白[134]生长,并产生丙酸,一种短链脂肪酸[135]。此外,Akkermansia通过粘蛋白降解支持仙人掌的生长,促进丁酸盐的生产[136]。如上所述,已知这些短链脂肪酸可增强肠道屏障功能。此外,据报道,在小鼠体内施用的阿克曼氏菌衍生的细胞外小泡定位于大肠,并通过增加紧密连接蛋白的上皮细胞表达直接增强肠屏障功能[137]。此外,给小鼠口服阿克曼菌可抑制高脂饮食诱导的粘蛋白层变薄,降低血LPS浓度,并抑制肥胖和异常糖代谢[138]。据报道,Akkermansia与肥胖(腰臀比和皮下脂肪细胞直径)和糖尿病(葡萄糖不耐受状态)呈负相关,并作为下一代益生菌引起了人们的关注[139]。在增加肠道菌群中阿克曼病比例的饮食因素中,多酚是一个有趣的因素,因为大多数干预研究使用多酚(苹果衍生的聚合原花青素、染料木素和异黄酮)或富含多酚的食品提取物(卡姆卡姆提取物、蔓越莓提取物和绿茶提取物)持续报告Akkermansia增加(表4)。Anhê等人报道,给小鼠服用蔓越莓提取物可增加结肠Kruppel样因子4(杯状细胞的标记物)和Muc2 mRNA的表达,表明多酚可促进粘蛋白的产生并支持阿克曼氏菌的生长[95]。另一方面,在一项使用人类肠道微生物生态系统模拟器(SHIME®)的研究中报告了多酚对阿克曼菌的直接益生元作用[140]。
Roseburia[141]是一种利用膳食纤维的肠道细菌,可通过产生丁酸增强肠道屏障。据报道,向小鼠施用玫瑰杆菌可增强肠道固有层中调节性T细胞的分化,并抑制肠道炎症[142]。如表4所述,据报道,低聚果糖、苹果衍生的聚合原花青素、海参衍生的硫酸多糖、卡姆卡姆提取物和灵芝菌丝体水提取物可增加肠道菌群中玫瑰杆菌的比例。Roseburia将低聚果糖代谢成果糖,用于生长,但在这个过程中,需要双歧杆菌产生的乙酸[143]。因此,为了通过摄入低聚果糖来生长蔷薇,有必要注意与其他肠道细菌的共生关系以及肠道中短链脂肪酸的含量。其他饮食因素,如原花青素、海参衍生的硫酸多糖、卡姆卡姆提取物和灵芝菌丝体,尚未对其对玫瑰杆菌的益生元功能进行研究。
有人认为,普雷沃杆菌的LPS具有较少的磷酸和酰基部分,有助于内毒素活性,从而导致比沙门氏菌的LPS具有更低的TLR4刺激能力[144]。因此,通过增加肠道菌群中普氏杆菌的数量,肠道内容物中的内毒素活性和对肠上皮细胞的损伤可能会降低,从而导致血液LPS水平降低。另一方面,Prevotella生产琥珀酸盐作为糖代谢的代谢物[145]。也有报道称,来自肠道细菌的琥珀酸被血清型鼠伤寒沙门氏菌[146]和艰难梭菌[147]利用并促进其生长,这是伪膜性结肠炎的病原体。据报道,琥珀酸通过琥珀酸受体诱发结肠炎,并促进结肠纤维化[148]。此外,据报道,2型糖尿病患者肠道菌群中普氏杆菌的比例与血液LPS水平呈正相关[149]。因此,普氏杆菌比例的增加并不一定对肠道健康产生积极影响。有必要仔细研究普氏杆菌对血LPS水平的影响。)
Clostridium, Escherichia, and Desulfovibrio are bacterial genera that may contribute to the increase of blood LPS levels. Many pathogenic bacteria (such as enterohemorrhagic Escherichia coli, Clostridium botulinum, Clostridium tetani, and Clostridium perfringens), which produce effector proteins or enterotoxins that disrupt epithelial tight junction belong to these genera [150]. In addition, the endotoxin activity of LPS in non-pathogenic Escherichia is also higher than in Bacteroides, and an increased proportion of these Escherichia in enteric flora aggravate colitis [151]. Clostridium species catabolize cholic acid to deoxycholic acid for their growth [152]. It is reported that, in mice, deoxycholic acid increases intestinal permeability through the reduction of goblet cell number, suppression of mucin production, induction of low-grade inflammation, and suppression of tight junction protein (ZO-1) expression [153]. In terms of dietary factors that reduce Escherichia, there are many reports of sulfated polysaccharides (Table 4). We could not find any reports that suggested the direct inhibitory effect of sulfated polysaccharide on growth of Escherichia. On the other hand, it is suggested that Bacteroides, that can be preferentially grown in sulfated polysaccharide feeding, compete with Escherichia in the co-culture assay [127]. In order to elucidate the mechanism by which sulfated polysaccharides reduce the proportion of Escherichia, it is hoped to study focusing on the interaction between gut microbes. Among the dietary factors that reduce Clostridium, procyanidin is reported to decrease the growth of Clostridium in fecal batch culture [154]. The bactericidal activity of methanol extract of nopal against Clostridium has also been reported [155].
Desulfovibrio is a gram-negative, obligate anaerobe, sulfate-reducing bacterium. Desulfovibrio utilizes electrons supplied by the oxidation of lactic acid in the electron transport system of the respiratory chain, uses sulfuric acid as the final electron acceptor, and produces hydrogen sulfide as a metabolite [156]. Desulfovibrio is ubiquitous in the intestines of humans and mice. Of the studies that showed significant changes in the proportion of Desulfovibrio, most studies reported that the proportion was increased associated to the reduction of blood LPS levels (Table 4). However, it is also reported that proportions of Desulfovibrio increased in the colons of patients with ulcerative colitis [157] and has attracted attention as a pathogen of colitis. In addition, Xie et al. reported in mice that the increase of Desulfovibrio in feces was positively correlated with the increase of LPS levels in feces, liver, and blood [45]. Qui et al. reported that ingestion of a high-fat diet in mice increased fecal Clostridium and Desulfovibrio, and oral administration of these bacteria to the normal chow-fed mice increased fecal and blood LPS levels [158]. These reports suggest that Desulfovibrio plays an important role as a source of LPS in the intestine. Desulfovibrio also competes with Anaerostipes caccae for lactic acid produced by Bifidobacterium, and reduces butyric acid production by inhibiting the growth of Anaerostipes caccae [159]. In addition, as the coexistence of Desulfovibrio and Bifidobacterium inhibits the growth of Bifidobacterium [159], this suggests that the amount of acetic acid produced by Bifidobacterium might be also reduced. On the other hand, it has also been reported that oral administration of Desulfovibrio increases the amount of hydrogen sulfide in the intestinal tract and inhibits intestinal peristalsis [160]. Desulfovibrio is thought to play an important limiting role in increasing blood LPS levels by supplying LPS, decreasing intestinal barrier function due to reduction of short-chain fatty acid content, and prolonging retention time of intestinal contents due to inhibition of peristalsis (Figure 2). However, despite Desulfovibrio being an important target for metabolic endotoxemia, few dietary factors have been reported to reduce the proportion of Desulfovibrio (Table 1, Table 2, Table 3 and Table 4).
(
梭菌、大肠杆菌和脱硫弧菌是可能导致血液LPS水平升高的细菌属。许多致病细菌(如肠出血性大肠杆菌、肉毒梭菌、破伤风梭菌和产气荚膜梭菌)都属于这些属,它们产生破坏上皮紧密连接的效应蛋白或肠毒素[150]。此外,非致病性大肠杆菌中LPS的内毒素活性也高于类杆菌,这些大肠杆菌在肠道菌群中的比例增加会加重结肠炎[151]。梭状芽孢杆菌将胆酸分解为脱氧胆酸供其生长[152]。据报道,在小鼠中,脱氧胆酸通过减少杯状细胞数量、抑制粘蛋白产生、诱导低度炎症和抑制紧密连接蛋白(ZO-1)表达来增加肠道通透性[153]。关于减少大肠杆菌的饮食因素,有许多关于硫酸多糖的报告(表4)。我们没有发现任何报告表明硫酸多糖对大肠杆菌的生长有直接抑制作用。另一方面,有人认为,在共培养试验中,可优先在硫酸多糖喂养中生长的类杆菌与大肠杆菌竞争[127]。为了阐明硫酸多糖降低大肠杆菌比例的机制,希望重点研究肠道微生物之间的相互作用。在减少梭菌的饮食因素中,据报道原花青素可减少粪便分批培养中梭菌的生长[154]。还报告了nopal甲醇提取物对梭菌的杀菌活性[155]。
脱硫弧菌是一种革兰氏阴性专性厌氧硫酸盐还原菌。脱硫弧菌利用呼吸链电子传输系统中乳酸氧化提供的电子,使用硫酸作为最终电子受体,并产生硫化氢作为代谢物[156]。脱硫弧菌在人和小鼠的肠道中普遍存在。在显示脱硫弧菌比例发生显著变化的研究中,大多数研究报告,该比例的增加与血液LPS水平的降低有关(表4)。然而,也有报道称,溃疡性结肠炎患者结肠中脱硫弧菌的比例增加[157],并作为结肠炎的病原体引起了人们的注意。此外,Xie等人在小鼠中报告,粪便中脱硫弧菌的增加与粪便、肝脏和血液中LPS水平的增加呈正相关[45]。Qui等人报告说,在小鼠中摄入高脂肪饮食会增加粪便中的梭菌和脱硫弧菌,而在正常的周粮喂养小鼠中口服这些细菌会增加粪便和血液中的LPS水平[158]。这些报告表明,脱硫弧菌作为肠内LPS的来源起着重要作用。脱硫弧菌还与仙人掌厌氧菌竞争双歧杆菌产生的乳酸,并通过抑制仙人掌厌氧菌的生长减少丁酸的产生[159]。此外,由于脱硫弧菌和双歧杆菌共存抑制了双歧杆菌的生长[159],这表明双歧杆菌产生的乙酸量也可能减少。另一方面,也有报道称,口服脱硫弧菌会增加肠道内硫化氢的含量,并抑制肠道蠕动[160]。脱硫弧菌被认为在增加血液LPS水平方面发挥了重要的限制作用,通过供应LPS,由于短链脂肪酸含量减少而降低肠道屏障功能,以及由于抑制蠕动而延长肠道内容物的保留时间(图2)。然而,尽管脱硫弧菌是代谢性内毒素血症的一个重要靶点,但很少有饮食因素能降低脱硫弧菌的比例(表1、表2、表3和表4)。)
Sulforaphane (1-isothiocyanato-4-methylsulfinylbutane) is an isothiocyanate with an N=C=S functional group and is abundant in broccoli (especially the sprout) and other cruciferous vegetables as the precursor glucoraphanin. Sulforaphane is thought to play a role in plant protection through its antimicrobial action [161], induction of programmed cell death of infected tissue [162], and inhibition of insect feeding [163]. On the other hand, in humans and rodents, sulforaphane activates NF-E2-related factor 2 (NRF2), which induces expression of genes expressing antioxidant and detoxication enzymes, including phase II enzymes, and then exerts anti-cancer [164], anti-liver damage [165], and anti-depressive effects [166].
We found that dietary administration of broccoli sprout extract reduced blood LPS levels and attenuated obesity, glucose intolerance, hepatic steatosis, and inflammation in mice fed a high-fat diet [94]. We also reported that the proportion of Desulfovibrionaceae [upper taxa (family) of Desulfobivrio] was positively correlated with blood LPS levels, and that ingestion of broccoli sprout extract reduced Desulfovibrionaceae in cecal contents. Subsequently, Wu et al. also reported that broccoli powder reduced the proportion of Desulfovibrio in the large intestinal contents of mice [167]. They reported that the decrease in Desulfovibrio composition was negatively correlated with the activity of myrosinase-like activity, isothiocyanate content, and NAD(P)H:quinone dehydrogenase 1 (NQO1) in the colonic mucosa. Ingested glucoraphanin is metabolized by myrosinase-like enzymes in enteric bacteria, which then produce sulforaphane [168]. Since sulforaphane enhances NQO1 activity through activation of NRF2 [168], it is suggested that sulforaphane metabolized and formed from glucoraphanin in broccoli sprouts may have an inhibitory effect on Desulfovibrio (Figure 2). Sulforaphane has been reported to exert antibacterial activity against the Proteobacteria (Desulfovibrio belongs this phyla) [169], but its direct effect on Desulfovibrio is not well understood. It is hoped that the mechanism by which sulforaphane decreases the proportion of Desulfovibrio will be elucidated.
5. Conclusions
In this article, we summarized previous reports about the regulation of metabolic endotoxemia through dietary factors, focusing on gut microbiota. Although changes in the composition of Firmicutes and Bacteroides due to excessive fat intake have been reported to contribute to metabolic endotoxemia in many reports, the results differ between studies and between species, and further investigation is needed to find true pathobionts. Moreover, since human epidemiological studies have not found a correlation between fat intake and blood LPS levels, it is necessary to search for dietary factors other than fat that cause metabolic endotoxemia. Regarding dietary factors that improve metabolic endotoxemia, human intervention studies have focused on probiotics, prebiotics, polyphenols and dietary habits, and it has been reported that prebiotics, including oligosaccharides, are effective. On the other hand, few studies have evaluated the effects of dietary intervention on gut flora in humans. The development and popularization of next-generation sequencing has made it possible to comprehensively analyze the “fecal” microbiota in humans. On the other hand, as mentioned above, there are also mucin-adherent bacteria that are thought to be involved in metabolic endotoxemia (e.g., Akkermansia and Bacteroides). In a colitis mouse model, it has been reported that the bacterial flora in the mucin layer exhibits changes from 12 weeks before the onset of colitis, and that the mucin layer was thinned [170]. In this study, changes in the fecal flora occurred at the same time as the onset of colitis, indicating that the bacteria in the mucin layer play an important role in understanding the physiological state of the intestinal tract. However, although it is possible to collect mucin layer samples in animals, it is not easy to do so in humans, due to ethical and technical obstacles. In the future, if a method for collecting the mucin layer in a noninvasive manner is established in humans, the research field of metabolic endotoxemia can be further advanced. Then, it is expected that we will comprehensively understand the relationship between dietary factors, dysbiosis, and metabolic endotoxemia in humans by conducting human intervention studies and epidemiological studies with dietary surveys, gut microbiota analysis using next-generation sequencers and evaluation of blood LPS levels.
(莱菔硫烷(1-异硫氰酸酯-4-甲基亚磺酰丁烷)是一种具有N=C=S官能团的异硫氰酸酯,在西兰花(尤其是芽菜)和其他十字花科蔬菜中大量存在,其前体为葡糖苷。萝卜硫素被认为通过其抗菌作用[161],诱导受感染组织的程序性细胞死亡[162],以及抑制昆虫取食[163],在植物保护中发挥作用。另一方面,在人类和啮齿类动物中,莱菔硫烷激活NF-E2相关因子2(NRF2),诱导表达抗氧化酶和解毒酶(包括II期酶)的基因表达,然后发挥抗癌[164]、抗肝损伤[165]和抗抑郁作用[166]。
我们发现,在喂食高脂饮食的小鼠中,西兰花芽提取物的饮食给药降低了血LPS水平,减轻了肥胖、葡萄糖不耐受、肝脂肪变性和炎症[94]。我们还报告了脱硫弧菌科[脱硫弧菌属的上层分类群(科)]的比例与血液LPS水平呈正相关,摄入西兰花芽提取物可降低盲肠中脱硫弧菌科的含量。随后,Wu等人还报告说,西兰花粉降低了小鼠大肠内容物中脱硫弧菌的比例[167]。他们报道,结肠粘膜中脱硫弧菌成分的减少与芥子酶样活性、异硫氰酸盐含量和NAD(P)H:醌脱氢酶1(NQO1)的活性呈负相关。摄入的葡萄糖苷由肠道细菌中类似芥子酶的酶代谢,然后产生萝卜硫素[168]。由于莱菔硫烷通过激活NRF2[168]来增强NQO1活性,这表明莱菔硫烷在西兰花芽中代谢并由格拉芬形成,可能对脱硫弧菌有抑制作用(图2)。据报道,莱菔硫烷对变形菌(脱硫弧菌属该门)[169]具有抗菌活性,但其对脱硫弧菌的直接作用尚不清楚。希望能够阐明莱菔硫烷降低脱硫弧菌比例的机理。
5.结论
在这篇文章中,我们总结了以前关于通过饮食因素调节代谢性内毒素血症的报道,重点是肠道微生物群。尽管在许多报告中,由于脂肪摄入过多而导致的厚壁菌和类杆菌成分的变化已被报道为导致代谢性内毒素血症,但研究结果在不同的物种之间和不同的物种之间存在差异,需要进一步的调查以找到真正的致病菌。此外,由于人类流行病学研究尚未发现脂肪摄入与血LPS水平之间的相关性,因此有必要寻找导致代谢性内毒素血症的饮食因素,而非脂肪。关于改善代谢性内毒素血症的饮食因素,人类干预研究的重点是益生菌、益生元、多酚和饮食习惯,据报道,益生元(包括低聚糖)是有效的。另一方面,很少有研究评估饮食干预对人体肠道菌群的影响。下一代测序技术的发展和普及使得全面分析人类“粪便”微生物群成为可能。另一方面,如上所述,还有粘蛋白粘附细菌被认为与代谢性内毒素血症有关(如阿克曼菌和类杆菌)。在结肠炎小鼠模型中,据报道,从结肠炎发病前12周开始,粘蛋白层中的菌群发生变化,粘蛋白层变薄[170]。在这项研究中,粪便菌群的变化发生在结肠炎发病的同时,这表明粘蛋白层中的细菌在了解肠道生理状态方面起着重要作用。然而,尽管可以在动物身上采集粘蛋白层样本,但由于道德和技术障碍,在人类身上采集粘蛋白层样本并不容易。未来,如果能在人类身上建立一种非侵入性收集粘蛋白层的方法,代谢性内毒素血症的研究领域将得到进一步的发展。然后,通过开展人类干预研究和流行病学研究,包括饮食调查、使用下一代测序仪进行肠道微生物群分析以及评估血液LPS水平,我们有望全面了解人类饮食因素、生态失调和代谢性内毒素血症之间的关系。)
上面内容来自【6】
#############################################################################
下面摘自【5】
Particularly, the coccus strain Pediococcus pentosaceus(戊糖片球菌菌株) eliminates LPS to 43%

上面内容来自【5】
############################################################################
############################################################################
高脂饮食=生酮饮食 吗?
不等于,高脂饮食中采用的都是劣质脂肪。
生酮饮食采用的是优质脂肪。[8]
#############################################################################
随着人的衰老,肠道菌群发生一定程度的演替,即拟杆菌门和双歧杆菌等厌氧菌数量的减少和厚壁菌门数量的增多。[7]
#############################################################################
附录(常用概念)
| 概念 | 解释 |
| FODMAP | FODMAP(可发酵性的寡糖、双糖、单糖以及多元醇)是食品中的一类短链碳水化合物及糖醇,高FODMAP饮食可加剧肠易激综合征的症状,Journal of Clinical Investigation[IF:12.784]近期发表的由华人学者团队开展的研究对其背后的机制进行了探讨,发现高FODMAP饮食可使肠道菌群失调,肠内高LPS水平似乎是诱导肠道病症的主因,而食用低FODMAP饮食可降低LPS水平,从而缓解IBS症状。值得关注的研究,推荐阅读。 |
| LPS | Lipopolysaccharides |
| 益生菌 | 益生菌是一类有利于维持肠道菌群稳态的活的微生物制剂,通过补充一种或多种对人体有益的细菌(如双歧杆菌、乳酸菌等)从而起到稳定肠道菌群的作用。 |
| 益生元 | 益生元是一类人体较难消化的成分,可以促进结肠中部分有益菌的生长,对人体产生积极的生理作用。 |
| 合生元 | 合生元是一类将益生菌和益生元联合使用的制剂。肠内营养(enteral nutrition,EN)是指通过经口摄食或管饲途径补充营养素的支持性治疗。 |
LPS的营养支持(没有验证):
黄酮类
硫辛酸
南非醉茄
虾青素
小檗碱(黄连素)
越橘
生物素
洋甘菊(芹菜素)
丁香
姜黄素
表没食子儿茶素没食子酸酯(EGCG)
鱼油(DHA,EPA和DPA)
亚麻油(α-亚麻酸)
叶酸
谷氨酰胺
绿茶
####################################################################
Psoriatics have been found to have deficient bile acid production in the liver. One role of bile acids is to break down fats and endotoxins.(服用胆汁酸治疗银屑病)[13]
We don't have enough studies into the effect of antibiotics on psoriasis, but at least one study found that psoriasis could be cleared with a long-term regimen of azithromycin.(长期服用阿奇霉素)[13]
####################################################################
荷兰Winclove益生菌公司开发针对银屑病的补充剂(预计于2020年开始,预计需要大约12-18个月才能完成)称为AxisBiotix,
同时要求减少酒精摄入或补充ω3脂肪酸和维生素D,适当摄入绿茶[14]
####################################################################
炎症性肠病和/或克罗恩病与银屑病之间可能存在联系。克罗恩病患者会出现皮肤症状,包括银屑病样斑块,一项研究表明,通常用于治疗银屑病的药物对克罗恩病也有效 [15]
####################################################################
The causes of leaky gut
Evidence suggests many factors can contribute to leaky gut, including stress; eating pesticide-laden foods; and an overgrowth of bacteria in the small intestine (SIBO). This overgrowth can be caused by long-term use of antacids, antibiotic use, and eating a standard Western diet full of processed foods.(少吃加工食品,)
Leaky gut is controversial
Readers should keep in mind that while a growing number of physicians believe leaky gut is the underlying cause of many medical conditions, many do not. Leaky gut and its possible connection to arthritic disease is not part of most standard medical school curriculums.(目前肠漏理论依然有争议并且在大部分教科书上看不见)[16]
#####################################################################
Together with the reduced F. prausnitzii levels, the psoriasis patients had a significantly higher abundance of E. coli. It was apparent that patients with concomitant IBD and psoriasis had the greatest decrease of F. prausnitzii and increase of E. coli.(银屑病患者体内的大肠杆菌丰度明显较高,且prausnitzii杆菌水平降低。很明显,伴有IBD和银屑病的患者的prausnitzii杆菌减少最多,大肠杆菌增加最多。)[17]
######################################################################
普雷斯特博士强调,偶尔吃“治疗食品”是可以的,但它们不应该构成你饮食的大部分。
克利夫兰诊所称,添加糖、饱和脂肪、反式脂肪、ω-6脂肪酸和精制碳水化合物也会增加炎症。一些研究表明,如果你患有牛皮癣并且对麸质过敏,那么食用无麸质饮食可以减少牛皮癣并减少发作。
你应该多吃什么食物?
普雷斯特说,如果你患有银屑病,消炎食品,如水果、蔬菜、植物蛋白、鱼、坚果和种子,应该占你饮食的大部分。
她说:“让你的盘子里的植物向前生长,至少一半的盘子里装满水果和蔬菜,并选择一小部分植物蛋白或瘦肉动物蛋白。”。“水果和蔬菜是自然界的消炎剂,因为它们富含抗氧化剂和植物化学物质。”
她补充说,将饱和脂肪和反式脂肪换成更健康的选择,如橄榄油、鳄梨、冷水鱼、大豆和坚果。添加醋、香草和香料代替添加的盐,选择低脂乳制品。
相关:地中海饮食中你可以吃的110种食物
地中海饮食包括大量水果、蔬菜、健康脂肪和瘦肉,具有抗炎作用,可以减少皮肤上的牛皮癣。
普雷斯特博士说,水果、蔬菜和全谷物也富含纤维,纤维有助于喂养健康的肠道细菌。益生菌,包括酸奶和发酵食品,益生元,如水果和蔬菜,也能改善肠道细菌。保持健康的肠道细菌可以减少炎症,并可以减少银屑病的症状。
如果你的饮食没有达到应有的健康水平,那么改变永远不会太迟。普雷斯特博士说,健康饮食的改变可以在几周内改善你的银屑病症状。[18]
######################################################################
In a 2014 paper in the Journal of Gastroenterology, scientists created and visualized the creation of inflammation and intestinal gaps (i.e. leaky gut) in IBS patients after the introduction of wheat, yeast, milk, and soy.8.
In addition to that, the typical Western diet, one that is high in fat, refined sugar, and low in fiber, consistently increases intestinal permeability in animal models7.
Fructose solutions can produce the same result, which may be why those with “sensitive tummies” often respond well to low FODMAP diets. We also know that certain drugs, like antibiotics and NSAIDs, disrupt the normal microbiome and affect permeability.(在《胃肠病学杂志》2014年的一篇论文中,科学家们创造并可视化了炎症和肠道间隙(即肠道渗漏)的产生在肠易激综合征患者中引入小麦、酵母、牛奶和大豆后。
除此之外,典型的西方饮食,即高脂肪、高糖和低纤维饮食,在动物模型中持续增加肠道通透性7。
果糖溶液也能产生同样的结果,这可能就是为什么那些“敏感肚子”的人通常对低FODMAP饮食反应良好的原因。我们还知道,某些药物,如抗生素和非甾体抗炎药,会破坏正常的微生物群并影响渗透性。)[19]
######################################################################
自两个患者组的样本均显示粪球菌物种丰度相对减少,而来自PsA患者的样本也表现为阿克曼菌、瘤胃球菌和假丁酸菌显著减少。PsA患者粪便样本上清液显示sIgA水平升高,RANKL水平降低。脂肪酸分析显示PsA患者和银屑病患者粪便中己酸盐和庚酸盐含量较低。[20]
################################################################
later work by Norrlind et al. successfully correlated streptococcal pharyngitis (as
evidenced by pharyngeal swab culturing and serology) with guttate psoriasis as well as exacerbations
of stable chronic plaque psoriasis(Norrlind等人后来的研究成功地关联了链球菌性咽炎(as)
咽部拭子培养和血清学)证实为点滴状银屑病以及恶化
稳定型慢性斑块型银屑病[21]
Interestingly, Chen et al. demonstrated that mice fed Lactobacillus pentosus developed a milder
form of imiquimod-induced psoriasis when compared to mice fed with a vehicle control [65].
Moreover, it has been shown that mice fed with Lactobacillus pentosus have suppressed psoriasis-related
pro-inflammatory and Th17-associated cytokines, such as TNF-α, IL-6, IL-23, IL-17A, IL-17F,
and IL-22 (有趣的是,Chen等人证明了喂食戊糖乳杆菌的小鼠产生了更温和的反应
与喂食溶媒对照的小鼠相比,咪喹莫特诱发的银屑病的形式[65]。
此外,研究表明,喂食戊糖乳杆菌的小鼠可抑制银屑病相关疾病
促炎和Th17相关细胞因子,如TNF-α、IL-6、IL-23、IL-17A、IL-17F,
和IL-22[66]。乳酸杆菌在人类银屑病患者中的直接作用仍需进一步研究
接受调查。)
After adhering to a gluten-free diet for three months, patients were noted to
have a significantly decreased dermal Ki-67+ cell population (an indicator of cell proliferation) in
lesional skin.(坚持无麸质饮食)[21]
################################################################
银屑病患者肠道菌群多样性减少。[22]
################################################################
It is unknown whether increased gut permeability is an early event in the pathogenesis of psoriasis or the consequence of the disease.(不清楚肠道通透性增加是银屑病的原因还是致病结果)[23]
However, the results were heterogeneous, with some patients presenting significantly affected intestinal integrity, and others showing a properly functioning gut barrier.(一些银屑病患者的肠道功能异常,其余患者正常)[23]
##################################################################
有人服用了Alflorex益生菌后银屑病痊愈[24]
另外有人补充维生素D,镁以及omega3[24]
################################################################
One study found patients who received a daily oral dose of Lactobacillus paracasei NCC2461 exhibited decreased skin sensitivity, improved barrier function, and improved efficiency in preserving skin moisturizing agents. In a murine model, a milder form of psoriasis was observed following administration of Lactobacillus pentosus. Lactobacillus pentosus has been shown to suppress psoriasis associated pro-inflammatory Th17-associated cytokines. (项研究发现,每天口服剂量为 副干酪乳杆菌 NCC2461降低了皮肤敏感性,改善了屏障功能,提高了保湿剂的保存效率。在小鼠模型中 轻度银屑病 服用戊糖乳杆菌后观察到。戊糖乳杆菌 已经证明可以抑制银屑病相关的促炎性Th17相关细胞因子)[25]
################################################################
In psoriasis, the deposit of excess antigens in the skin is thought to be one possible trigger for the development of a plaque.(皮肤中存在过量抗原的沉积)
Focus on anti-inflammatory foods including wild fish and other sources of omega-3 fats, red and purple berries (rich in polyphenols), dark green leafy vegetables, orange sweet potatoes, nuts and seeds. Add anti-inflammatory herbs and spices, including turmeric (a source of anti-inflammatory curcumin), garlic, oregano, ginger, rosemary, holy basil and turmeric, as well as green tea to your daily diet. Eliminate inflammatory foods such as refined, omega-6 and inflammatory oils including corn, soy and safflower oils.(关注抗炎食品,包括野生鱼类和其他来源的欧米茄-3脂肪、红色和紫色浆果(富含多酚)、深绿色叶菜、橙色甘薯、坚果和种子。在日常饮食中添加抗炎草药和香料,包括姜黄(一种抗炎姜黄素的来源)、大蒜、牛至、生姜、迷迭香、罗勒和姜黄,以及绿茶。消除发炎性食物,如精制、欧米茄-6和发炎性油脂,包括玉米油、大豆油和红花油。)
Remove food sensitivities
A review of research from 2008 strongly recommended gluten-free for people with this condition and celiac disease, but not for people without a celiac diagnosis or gluten sensitivity.(去除麸质饮食)[26]
################################################################
Gut microbiota are mainly composed of four phyla, namely, Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria (Qin et al., 2010). The human gut microbiota is dominated by only 2 of them: the Bacteroidetes and the Firmicutes (˃98%), whereas Actinobacteria, Proteobacteria, and others are present in minor proportions (Eckburg et al., 2005). Gut microbiota provides its host with a physical barrier to pathogens by competitive exclusion, such as occupation of attachment sites, consumption of nutrient, and production of antimicrobial substances.[27](人类肠道有一个主要的微生物定殖表面,富含可被微生物用作营养物质的分子,使其成为定植的首选场所,因此定植最严重的器官是胃肠道;据估计,仅结肠就含有人体内70%以上的微生物(Ley等人,2006年)。肠道微生物群主要由四个门组成,即厚壁菌门、拟杆菌门、放线菌门和变形菌门(Qin等人,2010)。人类肠道微生物群仅由两种菌群控制:拟杆菌和厚壁菌(98%),而放线菌、变形菌和其他菌群的比例较小(Eckburg et al.,2005)。肠道微生物群通过竞争性排斥为宿主提供了抵抗病原体的物理屏障,如附着位点的占据、营养物质的消耗和抗菌物质的产生。它还刺激宿主产生各种抗菌化合物。)
########################################################################
She also consumed more prebiotic-rich foods like dandelion greens and Jerusalem artichoke. (蒲公英绿和耶路撒冷朝鲜蓟)[28]
#######################################################################
Low-grade inflammation is alarmingly widespread, affecting about 40% of people in Western countries. 【29】
One aspect of diet that is particularly heinous is sugar, a well-known pro-inflammatory agent. Anything with added sugar should be avoided, as should all refined carbohydrates — white rice, bread, pasta, pastries, etc. Inflammatory skin conditions like acne are highly prevalent in Western countries, something that has been attributed to the abundance of carbohydrates in the diet.
Also on the “avoid” list are refined cooking vegetable oils: corn, soya, sunflower oils. These omega-6-rich vegetable oils are notoriously pro-inflammatory and should be avoided as much as possible. When you cook your food in oil, use coconut oil, butter, or extra virgin olive oil, and avoid processed foods — they nearly always contain refined vegetable oils.
“Elevated n-6 intakes are associated with an increase in all inflammatory diseases, which is to say virtually all diseases.”
Next, aim for a diet loaded with both probiotics and prebiotics.
Fermented foods are a good source of probiotics. Good examples include live natural yogurt, kefir, sauerkraut, and miso.
Probiotics are naturally anti-inflammatory, so feed them well with prebiotics.
Some of the best sources of prebiotics are onions, leeks, garlic, chicory root, coconut, carrots, asparagus, yams, and the cruciferous vegetables broccoli, cabbage, and sprouts.
The role of the gut microbiome in influencing the health of the whole body has been the focus of much recent research, but it is not a new discovery. As long ago as 1911, the gastroenterologist Milton H. Mack wrote that acne and eczema could be traced to the digestive tract, which he called “this fountainhead of diseases”. Today it’s called the gut-skin axis. Listen to what it’s trying to tell you.
(饮食中特别令人发指的一个方面是糖,一种众所周知的促发炎剂。任何添加糖的食物都应该避免,所有精制碳水化合物都应该避免,如白米、面包、面食、糕点等。在西方国家,痤疮等发炎性皮肤病非常普遍,这是由于饮食中含有丰富的碳水化合物。
同样在“避免”清单上的还有精制食用植物油:玉米油、大豆油、葵花籽油。这些富含ω-6的植物油是出了名的促发炎剂,应该尽量避免使用。当你用油烹饪食物时,使用椰子油、黄油或特级初榨橄榄油,避免加工食品——它们几乎总是含有精制植物油。
n-6摄入量的增加与所有炎症性疾病(也就是说几乎所有疾病)的增加有关
下一步,目标是饮食中同时含有益生菌和益生元。
发酵食品是益生菌的良好来源。好的例子包括天然酸奶、开菲尔、酸菜和味噌。
益生菌具有天然的抗炎作用,因此给它们添加益生元。
一些最好的益生元来源是洋葱、韭葱、大蒜、菊苣根、椰子、胡萝卜、芦笋、山药以及十字花科蔬菜西兰花、卷心菜和芽菜。
肠道微生物组在影响全身健康方面的作用一直是最近许多研究的焦点,但这并不是一个新发现。早在1911年,胃肠病学家Milton H.Mack就写道,痤疮和湿疹可以追溯到消化道,他称之为“疾病的源头”。今天,它被称为肠道皮肤轴。听听它想告诉你什么。)
###################################################################
In both groups, Firmicutes were the most common detected phylum followed by Bacteroidetes and finally Actinobacterial phyla. High statistically significant difference was reported for the Firmicutes/ Bacteroidetes ratio between the psoriasis patients and the control group and showed statistically significant positive correlations with PASI. Actinobacterial count was significantly higher in the control group than in psoriasis patients and showed statistically significant negative correlations with PASI. It is believed that, there are fractions of the gut microbiota with the ability to counteract inflammation (Bacteroidetes and Actinobacterial), and others that are more prone to induce inflammation (Firmicutes) and the disturbed microbiome ratio may be the cause for inducing psoriasis.
(在这两组中,厚壁菌门是最常见的检测门,其次是拟杆菌门,最后是放线菌门。银屑病患者和对照组之间的厚壁菌/拟杆菌比率存在高度的统计显著性差异,并显示出与PASI的统计显著正相关。对照组的放线菌计数显著高于银屑病患者,且与PASI呈统计学显著负相关。据认为,肠道微生物群中有一部分具有抵抗炎症的能力(类杆菌和放线杆菌),而其他部分更容易诱发炎症(厚壁菌群),并且紊乱的微生物群比例可能是诱发银屑病的原因。)[30]
#########################################################
These findings reveal that patients with psoriatic skin and joint disease should consider changing to a healthier dietary pattern[31]
######################下面是论文[32]的百度翻译#######################
表1。银屑病皮肤与健康对照组的细菌丰度比较
银屑病的低丰度
银屑病高丰度
表皮细菌[10,12]
痤疮表皮菌[11]
链球菌[10,14]
葡萄球菌[14]
金黄色葡萄球菌[11]
棒状杆菌[14]
益生菌如何帮助牛皮癣?
益生菌是活的微生物,能够通过恢复微生物组的平衡而带来许多健康益处。越来越多的研究关注益生菌在预防和治疗某些皮肤病(如特应性皮炎)中的应用。[15]尽管很少有研究专门关注益生菌在银屑病中的应用,从理论上讲,益生菌疗法可能通过促进减少银屑病炎症的良好细菌来平衡人的促炎和抗炎免疫功能。
哪些益生菌有助于治疗牛皮癣?
益生菌中最常见的微生物有:[16]
乳酸杆菌
双歧杆菌
肠球菌
表皮菌
布拉迪酵母菌
许多关于口服益生菌(包括乳酸杆菌和双歧杆菌)的研究表明,这些益生菌有助于预防和治疗过敏性皮炎等炎症性皮肤病。[17]尽管关于哪些益生菌菌株有助于特异性治疗银屑病的研究有限(表2),一项针对26名银屑病患者的研究比较了口服益生菌婴儿双歧杆菌25634对银屑病促炎标志物的影响,发现与安慰剂和基线相比,治疗8周后C反应蛋白和肿瘤坏死因子(TNF)-α水平显著下降。[18]
此外,一项体内研究发现,口服戊糖乳杆菌GMNL-77可显著降低促炎细胞因子mRNA水平(IL-6、TNF-a和IL-23/IL-17A),并减少咪喹莫特诱导的银屑病样炎症小鼠的皮肤红斑和鳞屑。[19]
一例脓疱性银屑病患者对类固醇和甲氨蝶呤无反应的病例报告显示,在6个月内每天口服三次产孢乳杆菌治疗其急性病变在2周内无新病变复发。[20]
一项针对1206名银屑病患者的饮食习惯、干预措施和改变的调查发现,40.6%的受调查患者报告在饮食中添加益生菌后情况有所改善或完全消失。[21]
表2。银屑病使用益生菌的临床证据总结
著者
研究类型
调查结果
Groeger等人,2013[18]
RCT*
26名患者在8周后口服婴儿双歧杆菌25634可降低CRP和TNF-α
陈等,2017[19]
前瞻性对照研究
口服戊糖乳杆菌GMNL-77可降低咪喹莫特治疗小鼠的IL-6、TNF-a和IL-23/IL-17A
Vijayashankar等人,2012[20]
病例报告
口服产孢乳杆菌可减少脓疱性银屑病,并在6个月内预防复发
Afifi等人,2017[21]
调查
在1206例银屑病患者中,40.6%的患者在饮食中添加益生菌后症状有所改善或完全消失[32]
##################################################################
In the study, New York University researchers found that psoriatic arthritis sufferers had lower levels of specific types of bacterium including Akkermansia, Ruminococcus, and Pseudobutyrivibrio. Normally, these bugs help keep your immune system under control, but when levels dip too low, all bets are off.(纽约大学的研究人员发现银屑病性关节炎患者的特定类型的细菌水平较低,包括阿克曼菌、瘤胃球菌和假丁酸菌。)[33]
##################################################################
Interestingly, night-shift workers exhibited not only an increase in severity of psoriatic flares, but also an increased incidence of psoriasis, suggesting that shifting the circadian rhythm (i.e., sleep and diet) may be a risk factor for psoriasis[52](夜班工人银屑病容易加重)
Previous studies have reported the positive effects of low-energy diets, vegetarian diets, formula diet weight loss programs, gluten-free or very low-calorie carbohydrate-free diet.
For instance, 12-O-tetradecanoylphorbol-13-acetate (TPA), a known inflammatory signal transducer, can induce psoriasis-like skin lesions in mice, while lesions and proinflammatory cytokine expression were significantly reduced in TPA-induced psoriasis by tangerine-derived nutrient flavonoids: Nobiletin (Nob) and 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone (5-HPMF)[67]. In addition, obesity, a result of poor nutrition, has been shown to exacerbate the severity of psoriasiform dermatitis in imiquimod-induced rodent models[68]. (橘子衍生的营养类黄酮(Nobiletin,Nob)和5-羟基-6,7,8,3′,可显著降低损伤和促炎细胞因子的表达)
Previous research has shown evidence for biochemical skin barrier restoration through topical administration of solenopsin, a compound of fire ant venom chemically similar to ceramides, and its derivates by reducing inflammatory markers and improving acanthosis in KC-Tie2 mice, an established rodent-model of psoriasis[69].(提到了螺旋藻毒素)
Up to 10% of patients with inflammatory bowel disease (IBD) are diagnosed with psoriasis[74]. Patients with psoriasis have a 3-fold higher risk of developing Crohn’s disease as compared to the general population; and Crohn’s disease patients have a 7-fold higher risk of developing psoriasis[75]. Recently, Scher et al[76], using pyrosequencing, found that patients with psoriatic arthritis and patients with skin psoriasis had a decreased bacterial diversity and a reduced relative abundance of some bacterial taxa such as Akkermansia, Ruminococcus, and Pseudobutyrivibrio, as compared to healthy controls. Among the risk factors for psoriatic diseases summarized in Table 1, overall, the alteration of gut microbiota may translate into physiological consequences including poor regulation of intestinal immune responses that may then affect distant organ systems[77-85]. Given the gut microbiome’s influence on the Gut-Skin axis, probiotic supplementation may have a promising role in the management of psoriatic patients. On this point, Gueniche et al[77], in a randomized double-blind placebo-controlled clinical study, showed that oral supplementation with the probiotic strain Lactobacillus paracasei decreased skin sensitivity and increased the rate of barrier function recovery.(高达10%的炎症性肠病(IBD)患者被诊断为银屑病[74]。银屑病患者发生克罗恩病的风险是普通人群的3倍;克罗恩病患者患银屑病的风险高7倍[75]。最近,Scher等人[76]利用焦磷酸测序发现,与健康对照组相比,银屑病关节炎患者和皮肤银屑病患者的细菌多样性降低,某些细菌类群(如阿克曼菌、瘤胃球菌和假丁酸菌)的相对丰度降低。在表1总结的银屑病风险因素中,总的来说,肠道微生物群的改变可能转化为生理后果,包括肠道免疫反应调节不良,从而影响远端器官系统[77-85]。鉴于肠道微生物组对肠道-皮肤轴的影响,益生菌补充可能在银屑病患者的治疗中发挥有希望的作用。在这一点上,Gueeniche等人[77]在一项随机双盲安慰剂对照临床研究中表明,口服补充益生菌菌株副干酪乳杆菌可降低皮肤敏感性并提高屏障功能恢复率。)[34]
#######################################################################
One study showed that oral administration of Lactobacillus pentosus GMNL-77 (as a probiotic), in a
mouse model of psoriasis, reduced tumor necrosis factor-α and IL-23–IL-17 axis cytokines, which was
associated with significant decreased erythematous scaling lesions and epidermal thickening in
comparison to untreated control mice [26]. Another study showed that Lactobacillus rhamnosus
suppressed the expression of TNF‑α, IL‑6, and proinflammatory cytokines in the IL‑23/IL‑17 cytokine axis
[27]. Our findings are in agreement with the previous study on severe pustular psoriasis cases; that did not
respond to steroids, dapsone, and methotrexate. However, after beginning probiotic supplementation
(Lactobacillus sporogenes) three times per day, the psoriatic patients showed significant clinical
improvement within two weeks with almost complete remission after four weeks [29]. In a placebocontrolled study of psoriasis patients, Bifidobacterium Infantis supplementation for eight weeks led to
significantly decreased plasma levels of inflammatory C-reactive protein and TNF‑α in comparison with
the placebo group [20]. The authors proposed that in response to probiotic supplementation, levels of the
beneficial microbial inhabitant of the large intestine increased. These species are much less abundant in
the gut of psoriatic patients than in healthy ones. Butyrate, an SCFA that provides energy for colonocytes,
reduces oxidative stress, and exerts anti-inflammatory action by triggering regulatory T cells, thereby
conferring immune tolerance beyond the GI system(戊糖乳杆菌+丁酸盐)[35]
####################################################################
The Probiotic Plan
“One of the reasons many chronic diseases, including autoimmune diseases, are on the rise is increased intestinal permeability, or leaky gut,” Dr. Sinatra says. “This can lead to immune dysfunction, setting the stage for autoimmune diseases to develop and progress.” (Note: Leaky gut is a concept credited more by alternative-medicine practitioners than by mainstream doctors.)(饮食+草药+补充剂需要一起上)
Picking the Best Probiotic to Take
When you’re searching for a probiotic, look at the label to see the number of colony-forming units (CFUs). “While the optimal probiotic dose remains unknown, it’s generally recognized in the medical community that formulas should contain at least 100 million CFUs to be effective,” Sinatra says. “But doses can be as high as hundreds of billions. And for formulas that list their strength in milligrams, you want to look for one that contains at least 350 milligrams of probiotics.”
There are a number of different kinds of probiotics to choose from. Some people benefit from taking lactic-acid probiotics like Lactobacillus or Bifidobacterium, while others do better with yeast-derived probiotics like Saccharomyces boulardii or soil-based organisms like bacillus. For some people, a combination of all three probiotic types is ideal.
Remember that probiotics sold as dietary supplements are not regulated by the U.S. Food and Drug Administration (FDA). To ensure purity, read labels carefully, look for supplements that are rigorously screened for contaminants, and consult with your healthcare team.(乳酸菌或双歧杆菌等乳酸益生菌中获益,而其他人则从布拉迪酵母菌等酵母源性益生菌或芽孢杆菌等土壤微生物中获益,并且至少摄入一万单位)
#####################################################################
If you discover that you have SIBO or dysbiosis, it’s very important to balance your microbiome using antimicrobial herbs, antibiotics, probiotics, prebiotics and more. This, in turn, will help to regulate your immune system!(如果你发现自己患有SIBO或生态失调,使用抗菌药草、抗生素、益生菌、益生元等来平衡你的微生物群是非常重要的。)
Regularly consuming fermented foods like kimchi, sauerkraut, yogurt and kefir is a great way to get these helpful bacteria into your system. (35, 36)发酵食品和益生菌饮料
You can also take a probiotic supplement to get these beneficial bacteria if you’re not a fan of fermented foods. (37)
多吃发酵食品或者是益生菌
BOOSTING PREBIOTIC AND FIBER INTAKE
Healthy gut bacteria use prebiotics and fiber to survive, grow, thrive and make SCFAs. (38) Increasing fiber intakes can boost their levels and function in the gut within just a few days! (39)
Easy ways to increase fiber intake include loading up on fiber-rich foods, such as fruits and vegetables, or taking a fiber supplement. (37)
GETTING ENOUGH EXERCISE
Studies show moderate aerobic exercise can increase the health of your gut bacteria. (40) The introduction of a regular exercise routine has also been shown to be useful in easing psoriasis symptoms. (41, 42, 43)
SHIFTING TO A WHOLE FOODS DIET
Study after study shows that a western style diet rich in calories, fat, simple sugars, and salt and low in fruits and vegetables is harmful to healthy gut bacteria, while traditional diets rich in whole foods help these bacteria grow. (47, 48, 49, 50)
Multiple studies also show that putting individuals with psoriasis on a whole-food diet can improve their symptoms. (41, 42, 43)
THE IMPACT OF DIET ON THE IMMUNE SYSTEM
Diet plays a crucial role in regulating a healthy immune system and controlling psoriasis.
增加益生元和纤维的摄入有利于SCFA
加工食品转向全食品不仅可以提高纤维摄入量、健康肠道细菌数量、SCFA水平和组胺水平,还可以直接抑制Th17细胞。[37]
Advanced Glycation End Products (AGEs)
Advanced glycation end products (AGEs) form when foods are heated to extreme temperatures, making them a common molecule found in broiled or fried foods like chips, french fries, chicken nuggets, fish sticks, etc. (56, 57)
Similar to how SCFAs and histamine help Treg cells mature, AGEs have been found to help inflammation-promoting Th17 cells mature. (58) This suggests that eating lots of fried junk food can increase the number of damaging Th17 cells.
TH17 FUNCTION AND WHOLE FOODS
On the other hand, replacing junk food with whole foods can help to reduce the damage from Th17 cells.
Potassium
One nutrient rich in many whole foods that may play an important role in regulating immune function is potassium.
High levels of potassium trigger the kidneys to produce a group of molecules called glucocorticosteroids. (59)
These molecules are natural steroids that work just like the drugs doctors prescribe. They are powerful inhibitors of inflammatory chemicals. (60)
Bioactive Phytonutrients
Many whole plant foods contain phytonutrients that have helpful health benefits. Some of these benefits include preventing the effects of inflammatory chemicals on the body. (61, 62, 63)
Some of these anti-inflammatory phytonutrients have powerful effects against inflammatory chemicals known to be important in damaging psoriatic skin.
For example, there is a phytonutrient in turmeric root called curcumin that blocks the effects of the inflammatory chemical TNF-alpha, which plays a major role in promoting psoriasis. (64, 65) In fact, curcumin blocks TNF-alpha nearly as well as drugs we designed to block it. (66)
In addition to the anti-inflammatory nature of phytonutrients, polyphenols (a type of phytonutrient) also boost the health of your microbiome. If you’d like to learn more about that process, check out my article on the topic here.
Antioxidants
As their name suggests, antioxidants block the effects of oxidants.
Since one of the key chemicals Th17 cells use to damage skin cells are oxidants (called reactive oxygen species (ROSs)), antioxidants from fruits, vegetables, nuts, seeds, teas and spices may be helpful in reducing psoriasis symptoms. (18, 67, 68, 69, 70)
THE BOTTOM LINE:
The evidence suggests that a whole food diet rich in foods that help promote a healthy microbiome may help prevent and reduce the symptoms of psoriasis and other autoimmune diseases.
(晚期糖基化终产物(AGEs)
高级糖基化终产物(AGEs)是在食物加热到极端温度时形成的,使其成为薯条、薯条、鸡块、鱼棒等烧烤或油炸食品中常见的分子。(56,57)
与SCFA和组胺如何帮助Treg细胞成熟相似,AGEs也被发现有助于炎症促进Th17细胞成熟。(58)这表明吃大量油炸垃圾食品会增加破坏性Th17细胞的数量。
TH17功能与全食
另一方面,用全脂食品代替垃圾食品有助于减少Th17细胞的损伤。
钾
钾是许多全食中富含的一种营养素,可能在调节免疫功能方面发挥重要作用。
高水平的钾触发肾脏产生一组称为糖皮质激素的分子。(59)
这些分子是天然类固醇,就像医生开的药一样有效。它们是炎症化学物质的强力抑制剂。(60)
生物活性植物营养素
许多全植物食物含有对健康有益的植物营养素。其中一些好处包括防止炎症化学物质对身体的影响。(61, 62, 63)
这些抗炎植物营养素中的一些对炎症化学物质具有强大的作用,已知这些化学物质对银屑病皮肤的损害很重要。
例如,姜黄根中有一种叫做姜黄素的植物营养素,它可以阻止炎症化学物质TNF-α的作用,TNF-α在促进银屑病方面起主要作用。(64,65)事实上,姜黄素对TNF-α的阻断作用几乎和我们设计的阻断TNF-α的药物一样好。(66)
除了植物营养素的抗炎性质外,多酚(一种植物营养素)还可以促进微生物群的健康。如果你想了解更多关于这个过程的信息,请查看我在这里的文章。
抗氧化剂
顾名思义,抗氧化剂可以阻止氧化剂的作用。
由于Th17细胞用来损害皮肤细胞的关键化学物质之一是氧化剂(称为活性氧物种(ROSs)),因此来自水果、蔬菜、坚果、种子、茶和香料的抗氧化剂可能有助于减轻银屑病症状。(18, 67, 68, 69, 70)
底线是:
证据表明,全食物饮食富含有助于促进健康微生物群的食物,可能有助于预防和减少银屑病和其他自身免疫性疾病的症状。)[37]
######################################################################################
[38]
The liver plays an important physiological role in lipopolysaccharide (LPS) detoxification and, in particular, hepatocytes are involved in the clearance of endotoxin of intestinal derivation. In experimental shock models, tumor necrosis factor (TNF)-alpha induces hepatocyte apoptosis and lethal effects are due to secreted TNF-alpha and not to cell-associated TNF-alpha. An exaggerated production of TNF-alpha has been reported in murine viral infections, in which mice become sensitized to low amounts of LPS and both interferon (IFN)-gamma and IFN-alpha/beta are involved in the macrophage-induced release of TNF-alpha. The prominent role of LPS and TNF-alpha in liver injury is also supported by studies of ethanol-induced hepatic damage. In humans, evidence of LPS-induced hepatic injury has been reported in cirrhosis, autoimmune hepatitis, and primary biliary cirrhosis and a decreased phagocytic activity of the reticulo-endothelial system has been found in these diseases. The origin of endotoxemia in hepatitis C virus (HCV) infected patients seems to be multifactorial and LPS may be of exogenous or endogenous derivation. In endotoxemic HCV-positive patients responsive to a combined treatment with IFN-alpha/ribavirin (RIB), endotoxemia was no longer detected at the end of the therapeutic regimen. By contrast, 48% of the non-responders to this treatment were still endotoxemic and their monocytes displayed higher intracellular TNF-alpha and interleukin (IL)-1beta levels than responders. Moreover, in responders, an equilibrium between IFN-gamma and IL-10 serum levels was attained. In the non-responders, serum levels of IL-10 did not increase following treatment. This may imply that an imbalance between T helper (Th)1 and Th2 derived cytokines could be envisaged in the non-responders.
(LPS会导致肝损伤)
##########################################################################
Compared with the stool samples of healthy controls, several major bacterial species were underrepresented or absent in the gut microbiota of psoriatic arthritis patients. These included Akkermansia, the most common mucolytic bacterium in healthy subjects. Intriguingly, Akkermansia counts are decreased 15-fold in Crohn’s disease and 92-fold in ulcerative colitis, according to the rheumatologist.
Other bacterial species markedly less abundant in the psoriatic arthritis patients’ gut flora were Ruminoccocus, Alistipes, and Roseburia. Like Akkermansia, these are mucin-degrading bacteria that promote a healthy gut environment, and they, too, are reduced in inflammatory bowel disease, Dr. Scher said.(银屑病关节炎患者肠道菌群中明显不太丰富的其他细菌种类有瘤胃菌群、阿利斯蒂普斯菌群和Roseburia菌群。Scher博士说,与Akkermansia一样,这些是粘蛋白降解细菌,可促进肠道健康,在炎症性肠病中也会减少)[39]
##########################################################################
The food we eat comes into direct contact with our gut microbiota so the macronutrients, vitamins, minerals, fibre, microorganisms and plant bioactive compounds from our diet are incredibly important.
But of particular note is the role of a cuppa when it comes to sorting our gut microbiota. New research reveals that four to five cups of tea per day could boost ‘friendly’ gut bacteria. The good news is that a range of different teas including black tea, green tea, oolong and pu-erh, can contribute to a positive shift in our gut microbiota. This positive shift can help avoid gut dysbiosis, in which gut bacteria composition becomes detrimental.
And the secret lies in tea’s rich array of polyphenols – natural flavonoid compounds found in the tea plant. Tea is one of the richest sources of flavonoid polyphenols in the diet of many global diets, demonstrating a prebiotic effect and rebalancing our gut microbiota towards more favourable strains including Lactobacillus, Faecalibacteria and Bacteroides.
A 10-day study where participants drank four to five cups of green tea daily instead of water, also showed increased levels of Bifiobacterium, a healthy bacteria strain.
Studies further proved that green and black tea polyphenols increased Bacteriodetes phyla and reduced Firmicutes. Green, oolong, black and pu-erh teas also increased bacterial diversity; important for strengthening immunity – vital for those with psoriasis – and also something particularly relevant in the current global situation.
Tea cup surrounded by tea leaves
Foods to help psoriasis
A change in diet can have an effect in as little as one or two days6 – so it’s worth eating more healthily from today.
High fibre diets have been linked with improved microbiota balance7.
Polyphenols are organic compounds found naturally in plants especially tea, fruits, vegetables, cocoa, wine, soya, spices and nuts. In general8, polyphenol-rich diets boost levels of beneficial bacteria and decrease more harmful species. Tea is one of the main sources of polyphenols in the British diet.
Vitamin A, vitamin D and long-chain omega-3 fatty acids all have roles in gut health.
Fermented foods and probiotic drinks contain live ‘good’ bacteria. If these are able to survive the pH extremes of the stomach and small intestine, the bacteria travel to the large intestine where they become established. A recent review9 examined evidence from 19 studies to assess the effect of fermented foods on the human gut microbiota. Promising findings were reported, but no overall conclusion.
Prebiotics are non-digestible carbohydrates, which can change the gut microbiota towards healthier species. They are found in various fruit and vegetables, such as tomatoes, onions, garlic, leeks, asparagus and bananas. Recent evidence suggests that tea can now also be considered a prebiotic. Prebiotics are relatively stable and, unlike probiotics, can be relied on to arrive relatively unchanged in the gut, despite digestive enzymes.
Avoid ultra-processed foods, which tend to be low in fibre and high in fat. They have been linked with inflammation and gut dysbiosis10.
Treatment options
In addition to taking responsibility for improving our gut microbiota, there is also a wide range of effective treatments for psoriasis. These range from over-the-counter moisturisers and emollients to prescription creams, ointments and medicines.
Finding the most effective therapy is often a matter of trial and error and new products are being introduced all the time, such as medicated tapes with a fixed dose of steroid. It’s important to keep going back to your doctor, or dermatologist, for advice if you are not happy.
At home, try and ensure you eat a balanced diet and make two to three cuppas part of that daily diet to help good gut health.
If you need support with your gut health, use the advanced search to find a nutrition professional who’s right for you.
Dr Tim Bond is a chemist, natural health expert and tea scientist. Dr Tim is part of the Tea Advisory Panel, a novel health group that conducts scientific studies into the healthy role of black tea in diets.
重点翻译如下:
秘密在于茶叶中含有丰富的多酚——茶树中发现的天然类黄酮化合物。在许多全球饮食中,茶是黄酮类多酚最丰富的来源之一,显示出益生元效应,并使我们的肠道微生物群向更有利的菌株(包括乳酸杆菌、粪杆菌和类杆菌)重新平衡。
一项为期10天的研究显示,参与者每天喝四到五杯绿茶,而不是水,双歧杆菌(一种健康的细菌)的含量也有所增加。
研究进一步证明,绿茶和红茶多酚增加了细菌门,减少了厚壁菌门。绿茶、乌龙茶、红茶和普洱茶也增加了细菌多样性;这对增强免疫力很重要——对银屑病患者来说至关重要——也是当前全球形势下特别相关的事情。
被茶叶包围的茶杯
帮助牛皮癣的食物
饮食的改变只需一两天就能产生效果6,因此从今天开始,更健康的饮食是值得的。
高纤维饮食与改善微生物群平衡有关7。
多酚是天然存在于植物中的有机化合物,尤其是茶、水果、蔬菜、可可、葡萄酒、大豆、香料和坚果。一般来说,富含多酚的饮食可以提高有益细菌的水平,减少更多有害物种。茶是英国饮食中多酚的主要来源之一。
维生素A、维生素D和长链ω-3脂肪酸都对肠道健康有作用[40]
####################################################################
随着银屑病的改善,瘤胃球菌科、黄连球菌属和布劳提亚属的丰度显著降低,银屑病患者的丰度显著增加[41]
####################################################################
Psoriasis and the Microbiome
Studies have shown that individuals with psoriasis often have an unbalanced gut microbiome and fewer bacterial species. People with psoriasis display low levels of helpful bacteria such as Lactobacillus and Bifidobacterium, and increased levels of harmful species including E. coli and Salmonella. The gut dysbiosis in people with psoriasis could lead to more inflammation and activation of immune cells. All of these changes could ultimately affect the level of inflammation in the body.
Although scientists do not know if leaky gut syndrome occurs regularly during psoriasis, some evidence suggests a connection. Additional studies are required to confirm the presence of leaky gut syndrome during psoriasis. However, the evidence suggests that treatment options targeting the microbiome could be beneficial for some people living with psoriasis.
Probiotics for Psoriasis
Probiotics are beneficial live bacteria that support the microbiome — in other words, good bacteria. When people talk about using probiotics to treat psoriasis or another health condition, they are generally referring to foods or supplements containing these good bacteria. Foods containing probiotics include:(
研究表明,银屑病患者的肠道微生物组不平衡,细菌种类较少。银屑病患者的有益细菌(如乳酸杆菌和双歧杆菌)水平较低,有害物种(包括大肠杆菌和沙门氏菌)水平较高。银屑病患者的肠道生态失调可能导致更多的炎症和免疫细胞的激活。所有这些变化最终都会影响体内的炎症水平。
虽然科学家不知道银屑病期间是否经常发生肠漏综合征,但一些证据表明两者之间存在联系。需要更多的研究来证实银屑病期间是否存在肠漏综合征。然而,证据表明,针对微生物组的治疗方案可能对一些银屑病患者有益。
银屑病益生菌
益生菌是有益的活细菌,支持微生物群,换句话说,是好细菌。当人们谈论使用益生菌治疗牛皮癣或其他健康状况时,他们通常指的是含有这些有益细菌的食品或补充剂。含有益生菌的食品包括:
(酸奶、凯菲尔、坦佩、康普茶、酸菜)
In a separate study, people with severe pustular psoriasis were treated with the probiotic supplement Lactobacillus sporogenes(芽孢乳酸杆菌) three times daily. Many of the study participants showed significant improvement within two weeks of treatment, and some even achieved remission after four weeks.[42]
#########################################################################
These include Akkermansia muciniphila, which helps regulate glucose metabolism and immune activity, and Faecalibacterium prausnitzii, which is a main producer of butyrate, an anti-inflammatory short-chain fatty acid that is important in colon health.(生产丁酸盐的Faecalibacterium prausnitzii)
Certain bacterial species in the gut microbiome of humans have been shown to significantly influence our immune systems. For instance, bacterial species such as Akkermansia muciniphila and Faecalibacterium prausnitzii favor the growth of regulatory T cells, a cell type that has anti-inflammatory properties and protects against autoimmunity. In theory, a relative decline in these bacterial species within the gut could lead to a loss of their anti-inflammatory effects, acting as an environmental trigger for the development of inflammatory diseases, such as psoriasis, in certain individuals.
翻译
人类肠道微生物群中的某些细菌种类已被证明会显著影响我们的免疫系统。例如,粘液阿克曼菌(Akkermansia muciniphila)和prausnitzii粪杆菌(Faecalibacterium prausnitzii)等细菌有利于调节性T细胞的生长,调节性T细胞是一种具有抗炎特性和防止自身免疫的细胞类型。
Liao: Clinical trials have been done investigating the use of probiotic supplementation in treating psoriasis. In a double-blind, placebo-controlled, clinical trial, researchers found no significant improvement in psoriasis symptoms in patients administered a probiotic containing Lactobacillus. In another study, however, when used as an adjuvant to topical treatment, probiotic supplementation significantly improved psoriasis severity in patients. Probiotic supplementation with Bifidobacteria infantis has also been shown to decrease systemic concentrations of pro-inflammatory TNF-alpha and C-reactive protein in psoriasis patients. The evidence for the effect of antibiotics is mixed, with some studies showing a beneficial effect on psoriasis and others showing a worsening effect.(乳酸杆菌无效、双歧杆菌有效)
For instance, research suggests that the gut microbiome is further altered in psoriatic arthritis versus skin-only psoriasis patients, with more significant reductions in Akkermansia, Ruminococcus and Pseudobutyrivibrio species seen.(Akkermansia、瘤胃球菌和假丁酸菌物种显著减少)
上面内容来自【44】
################################################################
So how does that apply to psoriasis? Well, let me tell you about two patients.
I’ll never forget a four-year-old girl who once arrived in my office with psoriasis from head to toe. She had been suffering since the age of six months. She looked like a red, swollen, inflamed mess.
This poor girl was on immune-suppressing drugs to address her numerous symptoms and ended up in the hospital from a MRSA (staph) infection. Doctors had kept her on antibiotics for a month.
While in my office, this little girl had to use the bathroom. You can imagine my horror when I heard her scream when she urinated because her genitals were inflamed with psoriasis, as well. Just when I thought her suffering couldn’t be any worse…my heart broke for her.
Rather than utilize invasive, potentially harmful creams and other drugs (which clearly weren’t working anyway), I applied the Functional Medicine approach with this girl. I looked at her brief but exhaustive health history. She was born by C-section. She had leaky gut and abnormal gut flora because of a long history of taking antibiotics and steroids that created a yeast overgrowth.
My solution was simple but powerfully effective: Remove the bad and add the good.
We eliminated trigger foods like gluten. We cleaned up the bad yeast with an antifungal. We incorporated anti-inflammatories, healthy fats and supplements like probiotics, vitamin D, vitamin A and zinc to heal her skin. I trusted she would get better even though she was the worst case I had ever seen.
Two weeks later, this little girl’s father called. “Dr. Hyman,” he said, “my daughter’s skin has completely cleared.” My broken heart healed a little bit that day.
Now, let’s look at a patient on the other end of the spectrum. I took this same approach with a 56-year-old doctor who came to see me with psoriatic arthritis. Despite loving his work, he was about to quit his job as a surgeon in a Massachusetts hospital because he was tired, overweight and could no longer operate due to the joint pain . I told him we needed to fix his gut with an elimination diet, get rid of the parasites, and introduce the right nutrients.
Six weeks later, my patient was off his immune suppressive drugs and a long list of other drugs. He had no symptoms. His digestive symptoms went away, his skin cleared up, he lost weight, and he could return to work.
While tempting to label these two patients’ transformations as miracles, they weren’t. Rather, they highlighted the power of Functional Medicine.
Heal Psoriasis with these Strategies
While psoriasis often becomes linked with gluten intolerances, that isn’t always the case. The three biggest culprits are:
Gluten sensitivities
Yeast overgrowth in the gut
Heavy metals exposure
To address psoriasis, I remove these obstacles while restoring the body’s natural balance. It really becomes that simple. Take away the things that cause the problem and add those that ameliorate it.
With that approach, I’ve found these eight strategies can naturally heal psoriasis without steroids, creams and other invasive procedures.
Eat a whole food, anti-inflammatory diet. Focus on anti-inflammatory foods including wild fish and other sources of omega-3 fats, red and purple berries (rich in polyphenols), dark green leafy vegetables, orange sweet potatoes and nuts. Add anti-inflammatory herbs, including turmeric (a source of anti-inflammatory curcumin), ginger and rosemary to your daily diet. Eliminate inflammatory foods such as refined, omega-6 and inflammatory oils – including: corn, soy and safflower oils.
Remove food sensitivities. These include gluten and dairy.
Test for heavy metal toxicity. Mercury and other metals trigger or exacerbate psoriasis.
Fix your gut. Your gut plays a significant role in skin health. One study found intestinal permeability (or leaky gut) can contribute to psoriasis. Yeast overgrowth, abnormal gut flora and other gut issues can also trigger or exacerbate psoriasis. If you suspect these or other issues, work with an integrative practitioner to optimize your gut health. I often use prescription or herbal antifungals to treat the yeast.
Use the right supplements. Nutrients like fish oil, vitamin D and probiotics can help eliminate psoriasis. Also consider anti-inflammatory nutrients like quercetin, grape seed extract and rutin. Using UltraInflamX PLUS 360 as a meal replacement also helps many of my patients with inflammation. You can find these and other professional-grade supplements in my online store.(
那么这对牛皮癣有什么影响呢?好吧,让我告诉你两个病人的情况。
我永远不会忘记一个四岁的小女孩,她曾经来过我的办公室,从头到脚都得了牛皮癣。她从六个月大起就一直在受苦。她看起来像一团红肿发炎的烂摊子。
这个可怜的女孩当时正在服用免疫抑制药物来缓解她众多的症状,最后由于感染了葡萄球菌而住进了医院。医生让她连续服用抗生素一个月。
在我的办公室里,这个小女孩不得不上厕所。你可以想象当我听到她小便时的尖叫时我的恐惧,因为她的生殖器也患了牛皮癣。就在我认为她的痛苦不会更糟的时候……我为她心碎了。
我没有使用侵入性的、潜在有害的药膏和其他药物(显然无论如何都不起作用),而是对这个女孩采用了功能医学的方法。我看了她简短但详尽的健康史。她是剖腹产出生的。由于长期服用抗生素和类固醇导致酵母菌过度生长,她有肠道渗漏和肠道菌群异常。
我的解决方案很简单,但非常有效:去掉坏的,添加好的。
我们消除了谷蛋白等易引发过敏的食物。我们用抗真菌剂清除了坏酵母。我们加入了消炎药、健康脂肪和益生菌、维生素D、维生素A和锌等补充剂来治疗她的皮肤。我相信她会好起来的,尽管她是我见过的最糟糕的情况。
两周后,这个小女孩的父亲打电话来。“海曼医生,”他说,“我女儿的皮肤已经完全干净了。”那天我破碎的心愈合了一点。
现在,让我们看看另一端的患者。我对一位56岁的医生采取了同样的方法,他来看望我患有银屑病性关节炎。尽管热爱自己的工作,但他即将辞去马萨诸塞州一家医院外科医生的工作,因为他感到疲劳、超重,并且由于关节疼痛无法再进行手术。我告诉他,我们需要用消除性饮食来修复他的肠道,清除寄生虫,并引入正确的营养素。
六周后,我的病人停止服用免疫抑制药物和一长串其他药物。他没有任何症状。他的消化症状消失了,皮肤变干净了,体重减轻了,他可以重返工作岗位。
虽然很容易将这两名患者的转变称为奇迹,但事实并非如此。相反,他们强调了功能医学的力量。
用这些方法治疗银屑病
虽然银屑病通常与麸质不耐受有关,但情况并非总是如此。三大罪魁祸首是:
面筋敏感性
肠道内酵母过度生长
重金属暴露
为了解决牛皮癣,我在恢复身体自然平衡的同时消除了这些障碍。事情就这么简单了。去掉引起问题的东西,加上改善问题的东西。
通过这种方法,我发现这八种策略可以自然治愈牛皮癣,而无需类固醇、面霜和其他侵入性治疗。
吃全食,消炎饮食。关注抗炎食品,包括野生鱼类和其他来源的欧米茄-3脂肪、红色和紫色浆果(富含多酚)、深绿色叶菜、橙色甘薯和坚果。在日常饮食中添加抗炎草药,包括姜黄(一种抗炎姜黄素的来源)、生姜和迷迭香。消除发炎性食物,如精制、欧米茄-6和发炎性油脂,包括:玉米油、大豆油和红花油。
消除食物过敏。这些包括麸质和奶制品。
重金属毒性试验。汞和其他金属会引发或加剧牛皮癣。
治好你的肚子。你的肠道对皮肤健康起着重要作用。一项研究发现,肠道通透性(或肠道渗漏)可能导致牛皮癣。酵母过度生长、肠道菌群异常和其他肠道问题也会引发或加重银屑病。如果您怀疑这些或其他问题,请与综合从业者合作,优化您的肠道健康。我经常使用处方药或草药抗真菌药来治疗酵母菌。
使用正确的补充剂。鱼油、维生素D和益生菌等营养素有助于消除银屑病。还可以考虑抗炎素如槲皮素、葡萄籽提取物和芦丁。使用UltraInflamX PLUS 360作为膳食替代品也有助于我的许多炎症患者。你可以在我的网上商店找到这些和其他专业级的补充剂。
)
Exercise regularly. Regular exercise is a natural anti-inflammatory. One study found increased physical exercise along with dietary intervention reduced psoriasis severity in systemically treated overweight or obese patients with active psoriasis. You don’t have to go to the gym, run on a treadmill and pump iron to stay in shape. Just start moving around more. Go for walks with your friends or family. Go out and do some gardening. Play Frisbee in the park with your kids. Pick up a tennis racket and just knock a tennis ball around. Anything you can do to get out and move your body can be considered exercise. So don’t think that you absolutely have to go to the gym to get fit. Just use your body more.
Practice deep relaxation. Studies show chronic stress can influence the development and exacerbation of psoriasis. The proportion of psoriasis patients who believe stress affects their skin condition ranges from 37 to 78 percent, and researchers believe stress may worsen psoriasis severity and may even lengthen the time to disease clearance. Calming techniques such as yoga, deep breathing, biofeedback, massage or my UltraCalm CD can reduce stress and anxiety to promote relaxation.
Sleep for 8 hours every night. Studies show patients with psoriasis suffer from more sleep disturbances. While some situations require professional help, you can improve sleep quality and quantity by implementing my 19 sleep tips from this blog.
If you’re looking for a place to get started to eliminate psoriasis, start with food. Try The Blood Sugar Solution 10-Day Detox Diet, which is anti-inflammatory and removes gluten, dairy and other food sensitivities. I’ve had patients create amazing, lasting changes to skin conditions and other problems in just 10 days. With this approach you can heal from the inside out with nutrients, not drugs.
While you might also consider working with a Functional Medicine practitioner to address issues like mercury or yeast overload, The Blood Sugar Solution 10-Day Detox Diet makes an excellent way to start.(
经常锻炼。经常运动是一种天然的消炎药。一项研究发现,在接受系统治疗的超重或肥胖活动性银屑病患者中,增加体育锻炼和饮食干预可降低银屑病的严重程度。你不必去健身房,在跑步机上跑步,也不必用力熨斗来保持身材。开始多走动。和你的朋友或家人一起散步。出去做些园艺工作。和孩子们在公园里玩飞盘。拿起一个网球拍,把一个网球打过来就行了。你能做的任何运动都可以被认为是锻炼。所以不要认为你一定要去健身房健身。多用你的身体。
练习深度放松。研究表明慢性压力会影响银屑病的发展和恶化。认为压力会影响皮肤状况的银屑病患者比例在37%到78%之间,研究人员认为压力可能会加重银屑病的严重程度,甚至可能延长疾病清除的时间。瑜伽、深呼吸、生物反馈、按摩或我的超平静CD等镇静技术可以减轻压力和焦虑,促进放松。
每晚睡8小时。研究表明,银屑病患者睡眠障碍较多。虽然有些情况需要专业帮助,但你可以通过实施我在本博客中的19个睡眠技巧来提高睡眠质量和数量。
如果你正在寻找一个地方开始消除牛皮癣,从食物开始。尝试血糖溶液10天排毒饮食,这是消炎和消除面筋,乳制品和其他食物的敏感性。我曾让患者在短短10天内对皮肤状况和其他问题产生惊人、持久的变化。通过这种方法,你可以用营养而不是药物从内而外治愈。
同时你也可以考虑和一个功能性的医生一起解决像汞或酵母超载这样的问题,血糖溶液10天排毒饮食是一个很好的开始方法。)
上面内容来自【45】
################################################################
What are short-chain fatty acids?
One important function of microbes in the gut flora is the production of short-chain fatty acids via the break-down of complex carbohydrates in food [2,3]. Examples of short-chain fatty acids are propionate, acetate, and butyrate.(什么是短链脂肪酸?
肠道菌群中微生物的一个重要功能是通过分解食物中的复杂碳水化合物产生短链脂肪酸[2,3]。短链脂肪酸的例子有丙酸盐、乙酸盐和丁酸盐。)
Short-chain fatty acids are thought to:
Help maintain the balance in the gut between its inflammatory and regulatory functions [2](短链脂肪酸被认为是:
帮助维持肠道炎症和调节功能之间的平衡[2])
Anti-inflammatory effects
Certain microbes promote white blood cells which contribute to anti-inflammatory responses [8]
Short-chain fatty acids inhibit inflammation and regulate immune cells [3]
Increased intestinal permeability
Changes in gut flora may increase the permeability of the intestine with subsequent leakage of gut microbiota into the bloodstream and skin resulting in systemic or cutaneous inflammation [2,3]
Neurotransmitters
Gut organisms alter levels of neurotransmitters which may influence the skin [2].
The gut microbiome and atopic dermatitis
Some studies have observed that gut dysbiosis (microbial imbalance) precedes the onset of atopic dermatitis [3,11,12]. Although the relationship is not clear, there are distinct differences in the gut microbiome between people with and without atopic dermatitis.
Infants who go on to develop atopic dermatitis have been reported to have:
Lower colonic microbial diversity [12]
Altered gut levels of certain gut bacteria (Staphylococcus aureus, Bacteriodetes and Clostridia [2,11])
Lower levels of Bifidobacterium levels, which are inversely correlated with severity [7]
Lower levels of Bacteroides and certain mucin-producing bacteria (Akkermansia muciniphila, Ruminococcus gnavus, and Lachnospiraceae) [7,11].
The gut microbiome and acne
Observed changes in microbial composition between individuals with and without acne may be due to:
Interactions with the mammalian target of rapamycin (mTOR) pathway and its effects on the intestinal barrier and metabolic function [3]
Dysbiosis stimulated by psychological stress and diet resulting in systemic inflammation [3].
Other
Small studies have reported possible associations of alterations in the gut microbiome with rosacea, psoriasis, and wound healing [3,5,9].
What are probiotics, prebiotics, and synbiotics?
Probiotics are defined by the World Health Organisation (WHO) as “live microorganisms which when administered in adequate amounts, confer a health benefit on the host” [5,10]. The most commonly used bacteria in probiotics are Lactobacilli and Bifidobacteria [2,5].
Prebiotics are non-digestible fibres that stimulate certain beneficial bacteria in the gut. The most common of these are non-digestible oligosaccharides [2].
Synbiotics are a combination of probiotics and prebiotics [2].
What is the role of probiotics, prebiotics, and synbiotics in skin disease?
The main role of probiotics and prebiotics is to restore and encourage the production of beneficial microorganisms in the gut [2]. Research is focusing on their therapeutic potential and results to date have been conflicting [9].
The role of probiotic supplements in the prevention of atopic dermatitis is being investigated in pregnancy and infancy to determine if they can improve or help prevent skin disease [2,11]. Probiotics have not been shown to treat established dermatitis [5,12].
It has been reported that probiotics may lead to an improvement in acne by reducing inflammation and decreasing ceramide production [12].
(抗炎作用
某些微生物促进白细胞,从而促进抗炎反应[8]
短链脂肪酸抑制炎症并调节免疫细胞[3]
肠通透性增加
肠道菌群的变化可能增加肠道的通透性,随后肠道微生物群泄漏到血液和皮肤中,导致全身或皮肤炎症[2,3]
神经递质
肠道生物改变可能影响皮肤的神经递质水平[2]。
肠道菌群与特应性皮炎
一些研究发现,肠道生态失调(微生物失衡)先于特应性皮炎[3,11,12]。虽然这种关系尚不清楚,但患有和未患有特应性皮炎的人的肠道微生物组有明显的差异。
据报道,继续发展为特应性皮炎的婴儿有:
结肠微生物多样性较低[12]
某些肠道细菌(金黄色葡萄球菌、杆菌和梭菌[2,11]的肠道水平改变)
双歧杆菌水平较低,与严重程度呈负相关[7]
类杆菌和某些粘蛋白产生菌的水平较低(粘蛋白Akkermansia muciniphila、gnavus瘤胃球菌和Lachnospiraceae)[7,11]。
肠道微生物群与痤疮
有痤疮和无痤疮的个体之间观察到的微生物成分变化可能是由于:
与雷帕霉素(mTOR)途径哺乳动物靶点的相互作用及其对肠道屏障和代谢功能的影响[3]
心理压力和饮食刺激的失调,导致全身炎症[3]。
另外
小型研究报告了肠道微生物组的改变可能与酒渣鼻、牛皮癣和伤口愈合有关[3,5,9]。
什么是益生菌、益生元和合生元?
世界卫生组织(WHO)将益生菌定义为“活微生物,当给予足够数量的益生菌时,会给宿主带来健康益处”[5,10]。益生菌中最常用的细菌是乳酸杆菌和双歧杆菌[2,5]。
益生元是刺激肠道中某些有益细菌的不易消化纤维。其中最常见的是不易消化的低聚糖[2]。
合生元是益生菌和益生元的组合[2]。
益生菌、益生元和合生元在皮肤病中的作用是什么?
益生菌和益生元的主要作用是恢复和鼓励肠道中有益微生物的产生[2]。研究的重点是它们的治疗潜力,迄今为止的结果一直相互矛盾[9]。
益生菌补充剂在预防特应性皮炎中的作用正在妊娠期和婴儿期进行调查,以确定它们是否可以改善或帮助预防皮肤病[2,11]。益生菌尚未被证明能治疗已确定的皮炎[5,12]。
据报道,益生菌可以通过减少炎症和神经酰胺的产生来改善痤疮[12]。)
上面内容来自【47】
#######################################################################
希波克拉底讲过:"All disease starts in the gut.”"

Lessons from bypass surgery patients
In 1975, Dr. Ely himself discovered the “bypass surgery syndrome” (which later was renamed bowel-associated dermatosis-arthritis syndrome). Patients presented with papulopustules, arthralgia and hypersensitivity to bacteria (especially Streptococcus pyogenes, the same bug that causes strep throat)– all after a bypass surgery. Almost all these patients had antibodies to peptidoglycan (PG: well-conserved and essential component of the bacterial cell wall). Interestingly, his psoriasis patients also had high titers of PG antibodies!3 This became the basis of his hypothesis that psoriasis may have a similar mechanism that involved the gut.
旁路手术患者的经验教训
1975年,伊利博士自己发现了“旁路手术综合征”(后来更名为肠道相关皮肤病关节炎综合征)。患者出现丘疹脓疱、关节痛和对细菌过敏(尤其是化脓性链球菌,导致链球菌性咽喉炎的同一种细菌)——所有这些都是在搭桥手术后出现的。几乎所有这些患者都有肽聚糖抗体(PG:细菌细胞壁的高度保守和基本成分)。有趣的是,他的银屑病患者也有高滴度的PG抗体!这成为了他的假设的基础,即银屑病可能有一个类似的机制,涉及肠道。
Gut-skin relationship in psoriasis
It was later discovered that psoriatic skin lesions were also loaded with PGs and PG-specific Th1 cells4. PGs are shown to have similar effects in vivo as endotoxin. So, it makes sense that acute psoriasis and endotoxemia have similar symptoms: fevers, leukocytosis, increased capillary permeability, elevated liver enzymes, and decreased complement levels. Moreover, gut bacterial DNA and other bacterial markers are present in psoriatic blood at higher levels, suggesting a “leaky gut” in psoriasis. Fascinating!
Now, figure 1 below shows a picture from Dr. Ely’s first talk on this topic in 1981. In psoriasis, PGs are absorbed from the gut into the 1) portal circulation, causing liver damage and 2) systemic circulation and end up in the skin and joints– leading to psoriasis and psoriatic arthritis, respectively. Dr. Ely calls psoriasis a “cathartic” event, whereby the body increases skin turnover in order to shed the PGs that is responsible for the systemic inflammation. Whoa, what a concept! It makes sense, though, doesn’t it? So, how does Dr. Ely suggest we treat this disease?
Skin Gut Axis in Psoriasis
Figure 1: In psoriasis, peptidoglycans are absorbed from the gut into the 1) portal circulation, causing liver damage and 2) systemic circulation and end up in the skin and joints– leading to psoriasis and psoriatic arthritis, respectively.
Bile acid therapy
Psoriasis patients have bad liver and abnormal bile. They also have a significant reduction of bile acid in bile5. Why does this matter? Well, bile acids have the ability to split endotoxins into non-toxic fragments, and they are also bactericidal. So, what if you replete bile acids in psoriasis? In one study of 500 psoriasis patients who were treated with bile acid (dehydrocholic acid) for 1-8 weeks, 434 (78.8%) became asymptomatic, compared to only 24.9% who received only conventional therapy6. Bile acid treatment of acute psoriasis led to 95.1% complete recovery. In another study, treatment of liver disease with bile acids led to dramatic improvement in those who also had psoriasis7.
The magic protocol
Here you go. Dr. Ely says that brands don’t matter and most of these are very affordable. I looked it up, and each of the following should cost about $10 –20 per month.
For 4 months: Azithromycin 500mg for 4 days, with 10 days between doses. This treats pyogenes, which is elevated in psoriatic small intestines
Probiotics containing Saccromycesboulardii 500mg 3 times daily (consider probiotics when prescribing any antibiotic for that matter)
Bile acids with each meal (ex: Ox bile, Duozymes– anything is fine)
Bioflavanoids: Quercetin 500mg twice daily
Now, it’s important to discuss and encourage a low-fat diet that is high in high-fiber (vegetables and fruits) and low in meats. It’s imperative that patients avoid alcohol and any peppers while on bile acid therapy, which can interfere with its efficacy.
Here are before and after photographs after just one month after this therapy. I know, it’s remarkable! Oh, and p.s., Dr. Ely recommends oral vitamin D in psoriasis patients — 5000IU vitamin D a day.
Before and after bile acid therapy
Before and after acid bile therapy 2
Concluding thoughts
I’m not saying let’s ditch the pharmaceuticals. But when I have mentioned this therapy with my psoriasis patients (albeit a short list so far), every single one of them was so grateful and excited to try a modality that is holistic, easily accessible, and affordable. And your staff is happier because no prior authorizations are needed! Hippocrates implored healers to “Let food be thy medicine and medicine be thy food.” We don’t have to take out the prescription pad right away, every time. When as physicians we take a more integrative stance, we give our patients more options and empower them to make lifestyle changes that will bring healing from the inside out. Let’s do it!!
Haines Ely M.D. is a clinical professor of dermatology at U.C. Davis and spends his days with UC Davis residents at Mather Field VA Hospital in Sacramento. He discovered the bowel bypass syndrome in 1975, which began a lifelong study of the interconnection of the bowel and the skin. He has successfully treated hundreds of psoriasis patients with bile acids and citrus bioflavinoids, plu.
(
银屑病的肠皮肤关系
后来发现银屑病皮损也含有PGs和PG特异性Th1细胞4。PGs在体内的作用与内毒素相似。因此,急性银屑病和内毒素血症有相似的症状是有道理的:发烧、白细胞增多、毛细血管通透性增加、肝酶升高和补体水平降低。此外,银屑病患者血液中存在较高水平的肠道细菌DNA和其他细菌标记物,提示银屑病患者存在“肠道渗漏”。极有吸引力的
下面的图1显示了Ely博士1981年关于这个话题的第一次演讲的图片。在银屑病中,PG从肠道吸收进入1)门静脉循环,导致肝脏损伤和2)体循环,最终进入皮肤和关节——分别导致银屑病和银屑病性关节炎。伊利博士将银屑病称为“泻药”事件,身体通过增加皮肤更新来释放导致全身炎症的PGs。哇,多好的概念啊!不过这是有道理的,不是吗?那么,伊利博士建议我们如何治疗这种疾病呢?
银屑病的皮肤-肠道轴
图1:在银屑病中,肽聚糖从肠道被吸收进入1)门脉循环,导致肝损伤和2)体循环,最终进入皮肤和关节——分别导致银屑病和银屑病关节炎。
胆汁酸疗法
银屑病患者肝脏不好,胆汁异常。它们还能显著减少胆汁中的胆汁酸5。为什么这很重要?胆汁酸有能力将内毒素分解成无毒的片段,它们也有杀菌作用。那么,如果银屑病患者体内充满胆汁酸怎么办?在一项研究中,500名银屑病患者接受了胆汁酸(脱氢胆酸)治疗1-8周,其中434名(78.8%)患者无症状,而仅接受常规治疗的患者只有24.9%。胆汁酸治疗急性银屑病的痊愈率为95.1%。在另一项研究中,用胆汁酸治疗肝病可使患有银屑病的患者得到显著改善7。
魔法协议
干得好。伊利博士说,品牌并不重要,而且大多数品牌都非常实惠。我查了一下,下面的每一项每月大概要花10-20美元。
4个月:阿奇霉素500mg,连续4天,两次给药间隔10天。这是治疗化脓,这是提高在银屑病小肠
含有Saccromycesboulardii的益生菌,每日3次,每次500mg(在为此开抗生素处方时,请考虑益生菌)
每顿饭都含有胆汁酸(例如:牛胆汁、多菌酶——任何东西都可以)
生物类黄酮:槲皮素500mg,每日两次
现在,重要的是讨论和鼓励高纤维(蔬菜和水果)和低肉类的低脂饮食。在接受胆汁酸治疗时,患者必须避免饮酒和任何辣椒,这可能会影响其疗效。
这是治疗一个月后的前后照片。我知道,这太了不起了!另外,伊利博士建议银屑病患者口服维生素D——每天5000 IU维生素D。
胆汁酸治疗前后
酸性胆汁治疗前后2
结语
我不是说我们放弃药物。但当我在我的银屑病患者中提到这种治疗方法时(尽管目前只是一个简短的列表),他们中的每一个人都非常感激和兴奋地尝试了一种全面、容易获得和负担得起的治疗方法。您的员工更快乐,因为不需要事先授权!希波克拉底恳求治疗师“让食物成为你的药,让药物成为你的食物。”我们不必每次都马上拿出处方纸。当我们作为医生采取更为综合的立场时,我们会给患者更多的选择,让他们能够改变生活方式,从内到外带来治愈。让我们做吧!!
海恩斯·伊利医学博士是加州大学戴维斯分校的皮肤病临床教授,在萨克拉门托的马瑟菲尔德弗吉尼亚医院与加州大学戴维斯分校的居民共度时光。1975年,他发现了肠旁路综合征,从此开始了对肠道和皮肤相互联系的终身研究。他已经用胆汁酸和柑橘类生物类黄酮成功治疗了数百名银屑病患者。
)
上述内容来自【48】
#######################################################################
Reference:
【1】科研 | STTT: 生酮饮食改变菌群降低结肠3型淋巴细胞,缓解小鼠结肠炎|微生物群|结肠炎|菌群|饮食|科研|肠道|炎症|-健康界
【2】JCI:FODMAP饮食促进肠道炎症的机制是什么 | 热心肠日报
【3】肠道“坏菌”产物——脂多糖(LPS)的作用机制与干预方案
【4】14 Factors that May Reduce Lipopolysaccharides (LPS) - SelfHacked
【6】Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors | HTML
【8】高脂饮食,会伤害肠道吗?生酮饮食和肠道的关系 - 知乎
【11】]细菌外毒素与内毒素的主要区别是什么?
【12】名家专栏 | 日常生活中,灭活乳酸菌可减除食品中黄曲霉毒素
【13】The connection between psoriasis and the gut
【14】Skin-gut axis: SkinBioTherapeutics and Winclove partner on probiotics to treat psoriasis
【15】Gut Health and Skin: Exploring the Connection - Chris Kresser
【16】The Science Behind Leaky Gut, the Gut Microbiome, and Arthritis
【17】Psoriasis, more than a skin disease? The involvement of gut microbes – Atlas of Science
【18】New Research Says These Are the Foods You Should Avoid If You Have Psoriasis (and What You Should Eat Instead!)
【19】What Is The Connection Between Leaky Gut And Psoriasis?
【21】Psoriasis and Microbiota: A Systematic Review
【22】People with Psoriasis More Likely to Develop Certain Gut Disorders | Live Science
【23】Clinical Implications of Intestinal Barrier Damage in Psoriasis | JIR
【25】A Gut Microbiota Approach to Psoriasis
【26】Types of psoriasis and how your gut health could be the underlying cause
【28】How To Ease Your Skin Issue By Starting With Your Gut, From An MD
【29】Heal Your Gut, Heal Your Skin
【32】IMPACT OF PROBIOTICS ON GUT & SKIN MICROBIOME IN PSORIASIS
【33】[33The Surprising Connection Between Bacteria And Psoriatic Arthritis
【36】Probiotics and Psoriatic Arthritis | Everyday Health
【37】Does Imbalanced Gut Bacteria Influence Psoriasis?
【38】The role of the liver in the response to LPS: experimental and clinical findings - PubMed
【39】Gut-joint connection promising in psoriatic arthritis
【40】Is my psoriasis caused by the gut? - Nutritionist Resource
【41】Involvement of Gut Microbiota in the Development of Psoriasis Vulgaris | medRxiv
【42】Probiotics for Psoriasis: Gut Bacteria and Your Microbiome | MyPsoriasisTeam
【43】Title: Elucidating role of bacteria in psoriatic disease: from skin and gut perspectives.
【45】8 Strategies to Eliminate Psoriasis | Dr. Mark Hyman
【46】Probiotics can treat psoriasis, shows new clinical data from ADM(做广告,没啥用)
【47】The gut microbiome in skin disease | DermNet NZ
【48】
Healing the Gut to Treat Psoriasis? - Next Steps in Dermatology

























 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








