reading notes of《Artificial Intelligence in Drug Design》
文章目录
1.Introduction
- In describing the DMPK (drug metabolism and pharmacokinetic) properties of molecules it is recognized that these can be divided into absorption, distribution, metabolism, and elimination (ADME) and then subdivided into dependent and independent descriptors such as clearance (Cl), volume of distribution (Vd), fraction absorbed (fabs), bioavailability (F), and unbound fraction (fu).
- One of the key challenges to AI-based methods will be the ability to adapt to data sets that change in quality over time.
2.The Evolution of DMPK
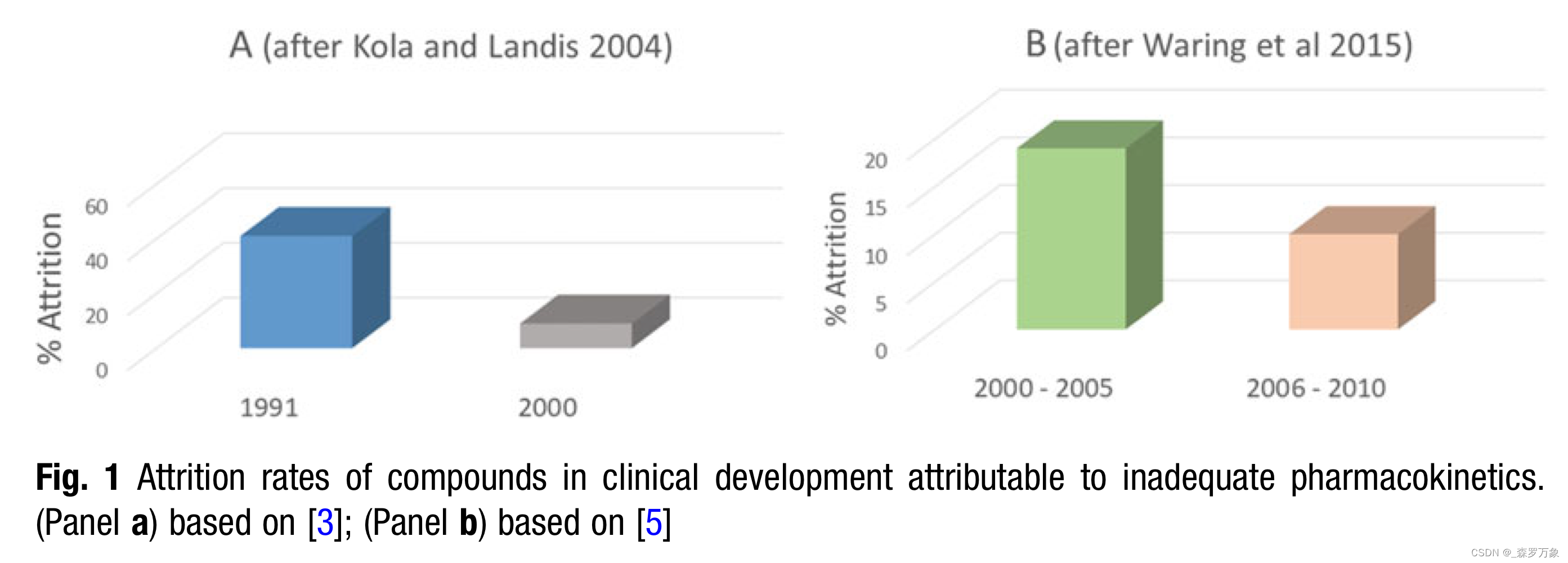
- The improvement in the ability of the pharmaceutical industry to predict human pharmacokinetic behavior can be loosely attributed the three major technological shifts from 1990 to the present day.
- The first major shift is the general availability of tandem mass spectrometers (LC/MS/MS) with tabletop footprints. The abundance and accessibility of LC/MS/MS has made sensitive and specific quantitation of drug candidates in virtually any biological matrix commonplace.
- Second, the widespread availability of high quality in vitro systems from both human and nonhuman sources has facilitated the development of in vitro–in vivo correlation and extrapolation.
- Finally, the availability of cheap computing power and memory has allowed the expansion and general usage of methods like physiologically based pharmacokinetic modeling (PBPK) and machine learning to researchers in general as opposed to individual, select specialists.
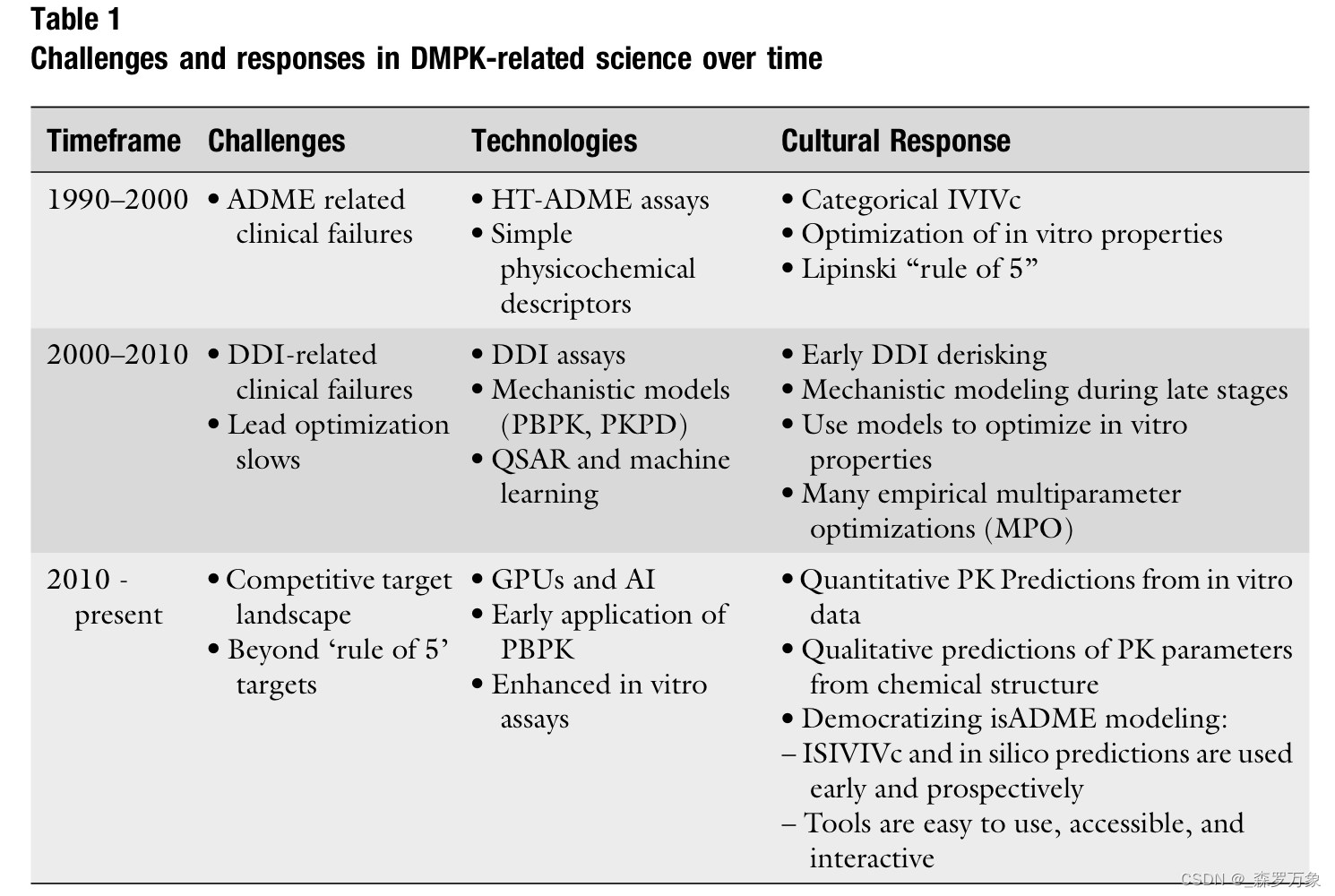
3.Opportunities for AI in PK Prediction
- As George Box noted, “All models are wrong, some are useful.”
- In the broadest sense, the opportunities for AI/ML in PK prediction can be divided into two general categories (Fig. 2).
3.1.Individual Parameters
- The application of the discrete estimate of ADME properties fall into two general categories within drug discovery.
- The most obvious is to track the progress of medicinal chemistry efforts toward improvement of a specific parameter in isolation.
- The second application of these parameter estimates would be to serve as input data, either alone or in combination to a parametric pharmacokinetic model. These parametric pharmacokinetic models could span from traditional compartmental models to physiologically based pharmacokinetic models (Fig. 3)
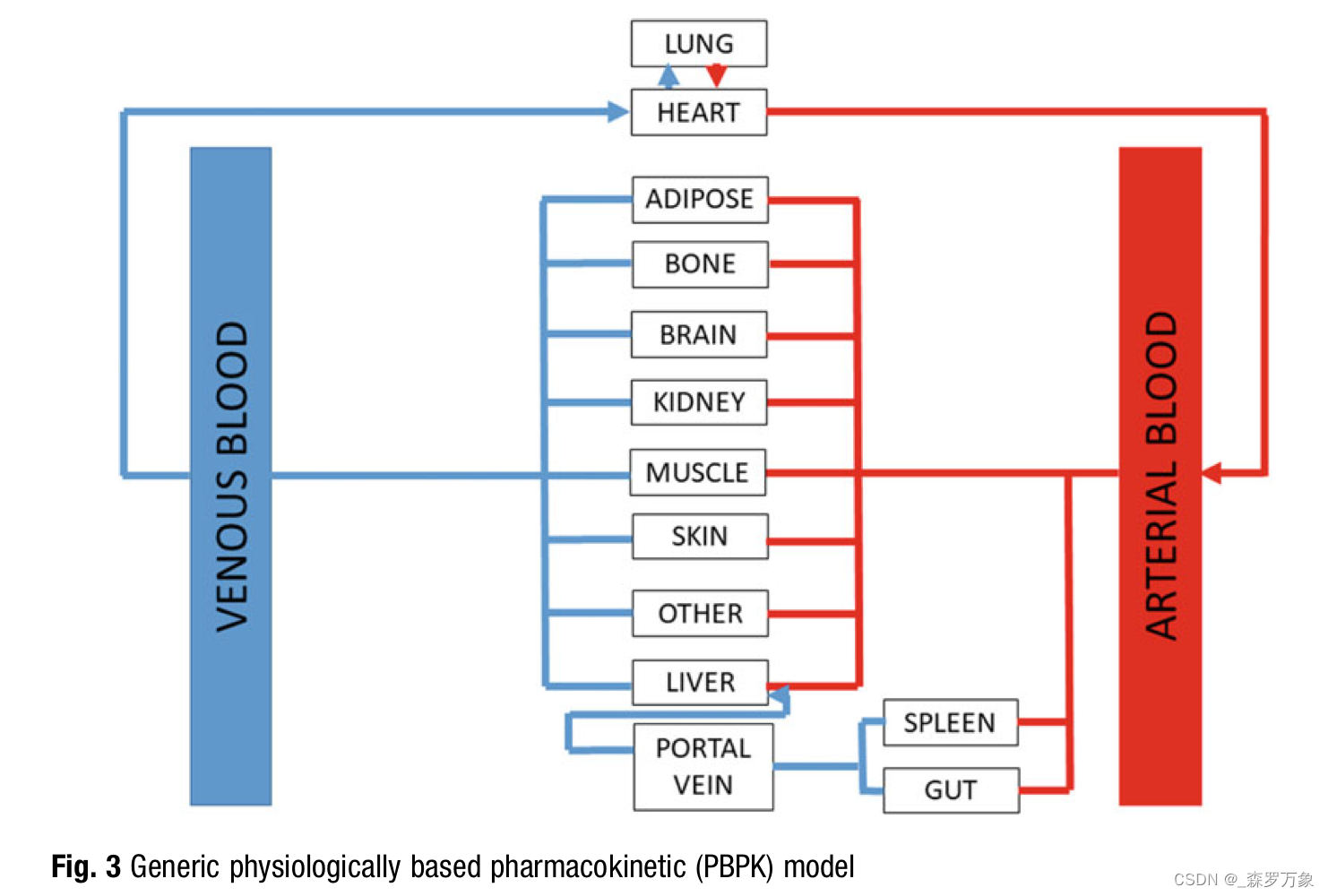
- The most obvious weakness of this individual parameter and parametric model approach is the very nature of its defined structure.
3.2.Overall Profile
- While apparently less well investigated than the previous approach, there is interest in the direct prediction of concentration profiles directly from input data without a formal, independent pharmacokinetic modeling step. The major factor that can drive this approach is the existence of large amounts of nonclinical pharmacokinetic data already gathered within companies.
- There are two very obvious drawbacks to the approach of predicting the full concentration-time profile directly.
- Indeed you may be able to predict the curve but would have no idea of the key drivers (e.g., fraction absorbed, clearance, and volume) that influence the shape of the curve.
- A second drawback is the as yet unknown ability to express the uncertainty around the estimates produced.
3.3.Quantitative Prediction vs. Categorization
- A primary limitation of broad classification systems is their reduction of what are essentially continuous variables in an in vitro assay to discrete, discontinuous values.
- As an example, consider the use of “stable” in the context of metabolic stability. Whether defined by a percent of parent remaining following an incubation, an apparent intrinsic clearance or an extrapolated in vivo clearance, the boundary is arbitrary, the use misleading as it does not and cannot mean no (zero) turnover, and frequently when used as an approximate of an in vivo hepatic clearance spans a range from 0% to 30% of hepatic blood flow.
3.4.Data Visualization
- Although there are conceptual similarities in this approach to multiparameter optimization (MPO) and scoring, our experi- ence suggests that a more direct connection to output that can be directly compared to experimental data has greater impact than ranking via an MPO score.
3.5.Life-Cycle Considerations
- There is significant potential for the parametric approach exemplified by PBPK to create a “learn and confirm” cycle that would allow the PBPK model to evolve over time and also feedback potential chemical alternatives for future synthesis.
4.Data Quality
- Although it is difficult to define quantitative descriptors of the “quality of datasets” strictly, there is clear value in evaluating data in terms of how the result informs what part of the result is a property of the compound and what part of the result is a condition of the experiment. In this, a property would be an attribute of the compound that is transferable between scenarios (e.g., Clint—intrinsic clearance) while a “condition of the experiment” would be that part of the observation that is an attribute of study design or execution (e.g., bioavailability in the fed vs fasted condition).
4.1.In Vitro Data
-
In vitro assays have the inherent ability
- to focus on specific mechanisms relevant to drug disposition and inform discrete parameters in nonclinical species.
- to create experimental systems from material derived from humans and nonclinical species and this provides rich opportunity for both transla- tion to the in vivo and further delineation of the discrete steps of the mechanism under study.
-
While we can frequently make discrete observations around an ADME property, these observations typically represent hybrid parameters that describe the rate-controlling step of the process described.
-
One of the most widely used in vitro assays, microsomal stability, provides a very rich example from which to illustrate the previously mentioned concepts.
-
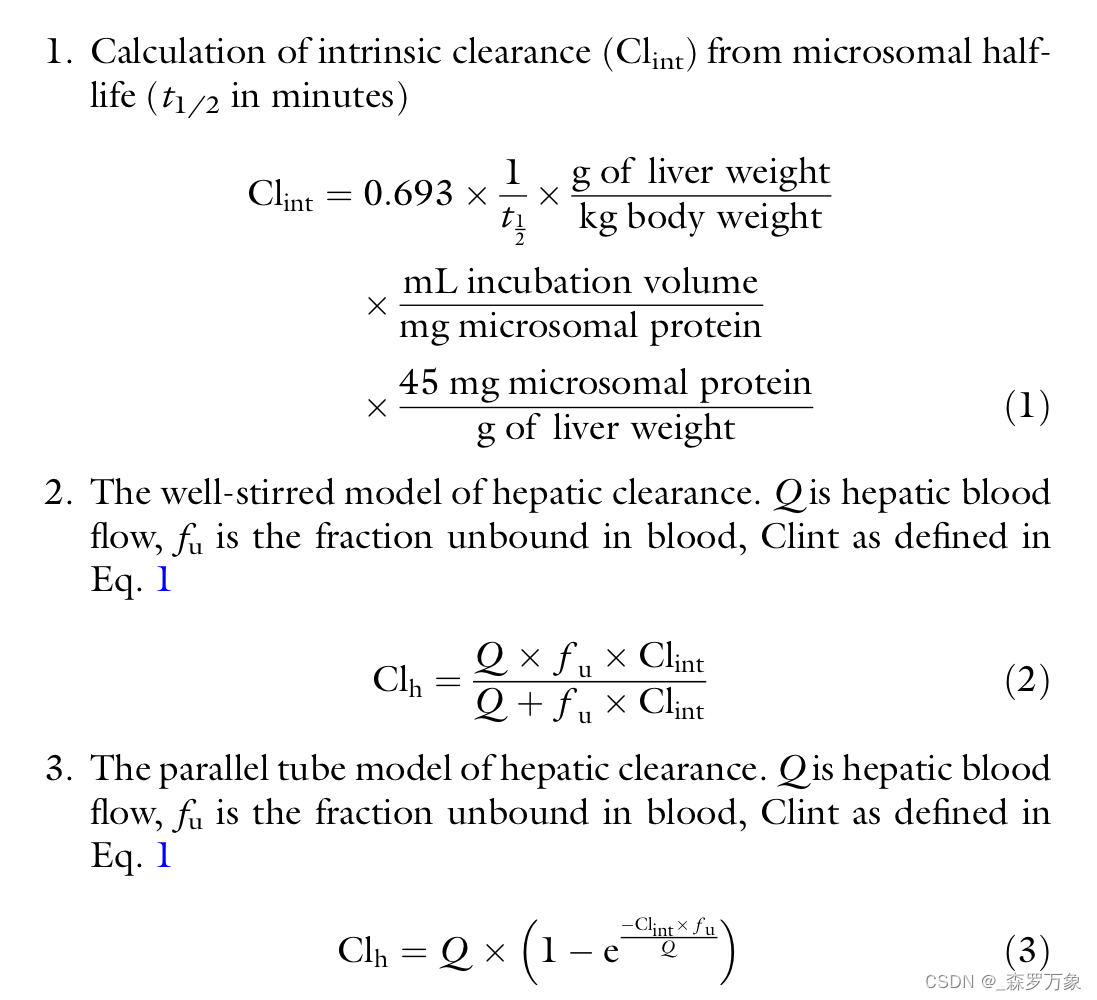
In practice, the application of the microsomal stability assay in predicting in vivo clearance involves the estimation of the apparent half-life from the disappearance curve of the compound. This half-life is multiplied by factors accounting for the protein content and volume of the incubation, the relative amount of microsomal protein in the liver and the ratio of the liver to total body weight and yields an value called intrinsic clearance (Clint) as illustrated in Eq. 1. This Clint value can then be converted into and estimation of total body clearance using either the approximation of the well-stirred or parallel tube models of hepatic clearance as shown in Eqs. 2 and 3.
-
While the simplicity and directness of this approach accounts for its broad application, they belie several underlying assumptions that predispose this assay to misinterpretation and the consequent presentation of spurious correlations. The in vitro turnover described in this process will be representative of that in vivo based on the following assumptions:
- (1) The liver is the main organ of clearance.
- (2) Metabolic clearance greatly exceeds renal and biliary clearances.
- (3) Oxidative metabolism is much great than other metabolism.
- (4) All drug in the incubation is freely available to the active site of the metabolic enzymes (i.e., the free fraction in the incubation is 1.
- (5) Substrate concentration is below the Km of the enzymes.
- (6) There is no inactivation of the enzymes.
- (7) Equilibrium is not approached.
-
Another practical aspect of metabolic stability assays is their reliance, at least in the drug discovery realm, on measuring parent disappearance. Since analytical assays have defined precision and accuracy associated with them as compounds become more stable and the fractional change between time points reduced, there will be a limit beyond which increasing stability cannot be defined. Lengthening the incubation time, particularly with microsomes is not an option given the assumptions around inactivation, substrate depletion and product inhibition mentioned previously. Simply increasing microsomal protein content is also not an option as the change in reaction rate frequently becomes nonlinear. A similar problem is encountered in hepatocyte assays and has resulted in the development of the “relay” assay method and coculture techniques (HepatoPac and Hurel) that extend the ability to differentiate stable compounds but do not fully resolve this issue. An alternative possibility would be to measure product formation rather than parent disappearance but this is largely impractical is a discovery setting.
-
the evolution of an assay over time, as this will create segments of data that may differ in quality. This situation is not unique to the microsomal stability assay as can be inferred from the comments around hepatocytes. Ultimately, this continuous refinement of assays is a general phenomenon independent of the specific assay discussed. The relevant principle to AI/ML built models is that the quality of the underlying data in terms of precision, accuracy and specificity may change over time and consequently data for compounds may not be equivalent.
5.In Vivo Data
- While correlation between predicted in vitro clearance and observed total body clearance can result in obscured understanding of changes in metabolic stability, a generic representation of the relationship between predicted hepatic clearance and observed clearance (Fig. 4) can be useful in illustrating some of the considerations in understanding the quality of pharmacokinetic data following intravenous administration.

- The delineation of quadrants I and III is arbitrary and in practice the types of deviation in quadrant II often go unaddressed which may be a challenge for future use in AI/ML approaches since this may also reflect bias within the dataset.
5.1.Observed Data as the “Gold Standard”
- The nature of the observed concentrations are that they are experimentally determined and consequently subject to a variety or error. It is likely that little can be done to address these concerns a priori but they may be worthwhile considerations in evaluating AI/ML output and managing expectations around the accuracy of their predictions.
6.Opportunities and Future Challenge
6.1.Mechanism vs. Empiricism
- The future opportunity this may create is that the difference between the predictions of the parametric and empirical models may inform how much of the biology is not understood and may point the way for further refinement of the parametric model and/or suggest new in vitro assays to describe the mechanistic possibilities underlying the discrepancies.
6.2.New Modalities
- Much of the data existing describes disposition at extreme ends of chemical space: small molecules (<1500 Daltons) or large biologics (>50,000 Daltons). Between these limits is relatively unexplored chemical space for which we do not yet understand the principles governing disposition. Given the high degree of interest in this portion of chemical space, significant opportunities exist to not only model this space, but to connect it to the extremes on either side.
7.Forward-Looking Perspective
- Since our understanding of the underlying biology of the in vitro and in vivo systems with respect to ADME, one of the key challenges to AI-based methods will be the ability to adapt to data sets that change in quality over time. In addition, the ability to demonstrate the benefits derived from AI-based methodology raises many interesting questions. If drug approval rates are the metric by which improvement is measured, the relevance is clear but the period and numbers of approvals required to show a benefit may be impractical. The identification of appropriate comparator methods should be a focus of activity.
























 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?










