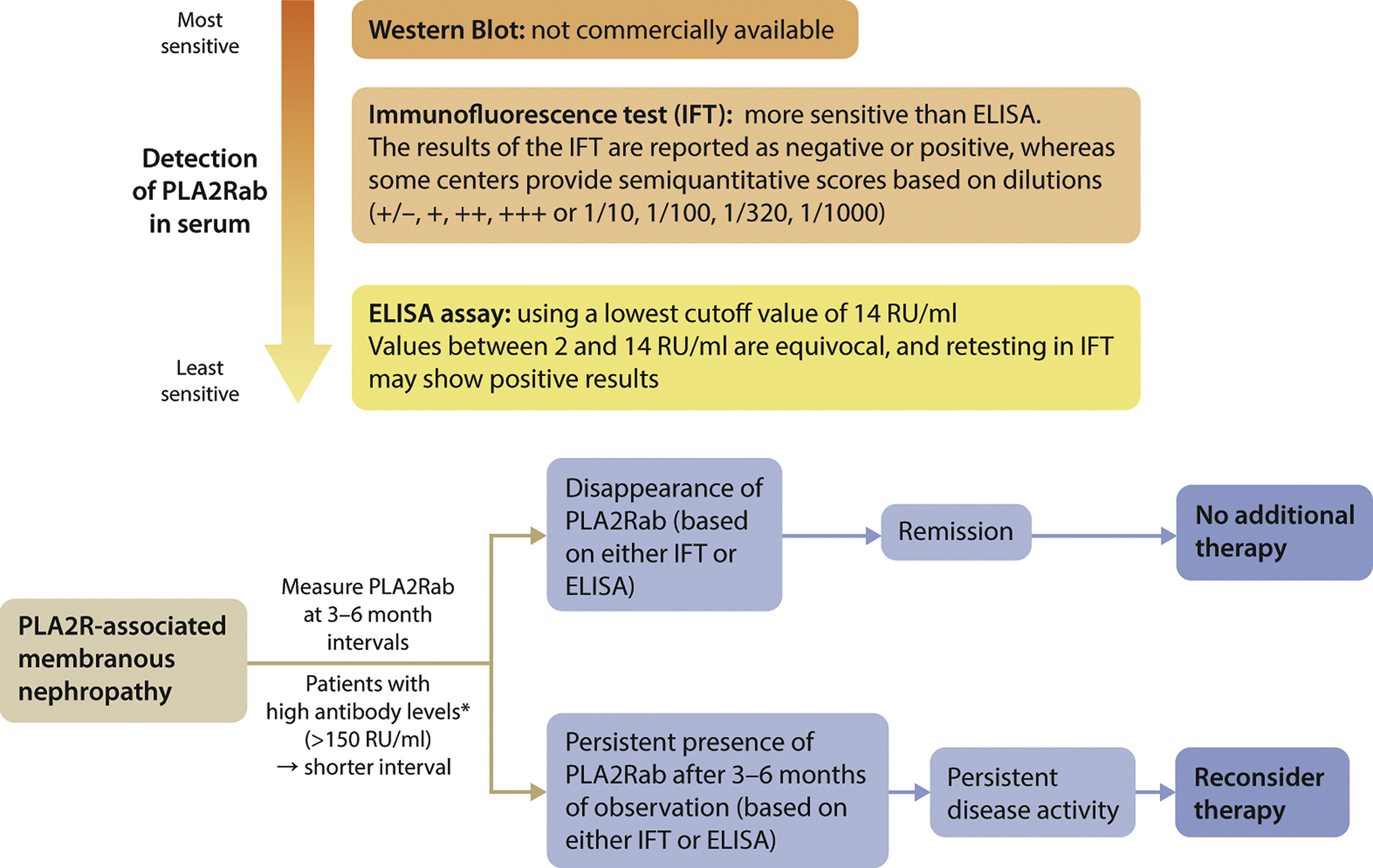
ↈ anti-PLA2R antibody 的检测
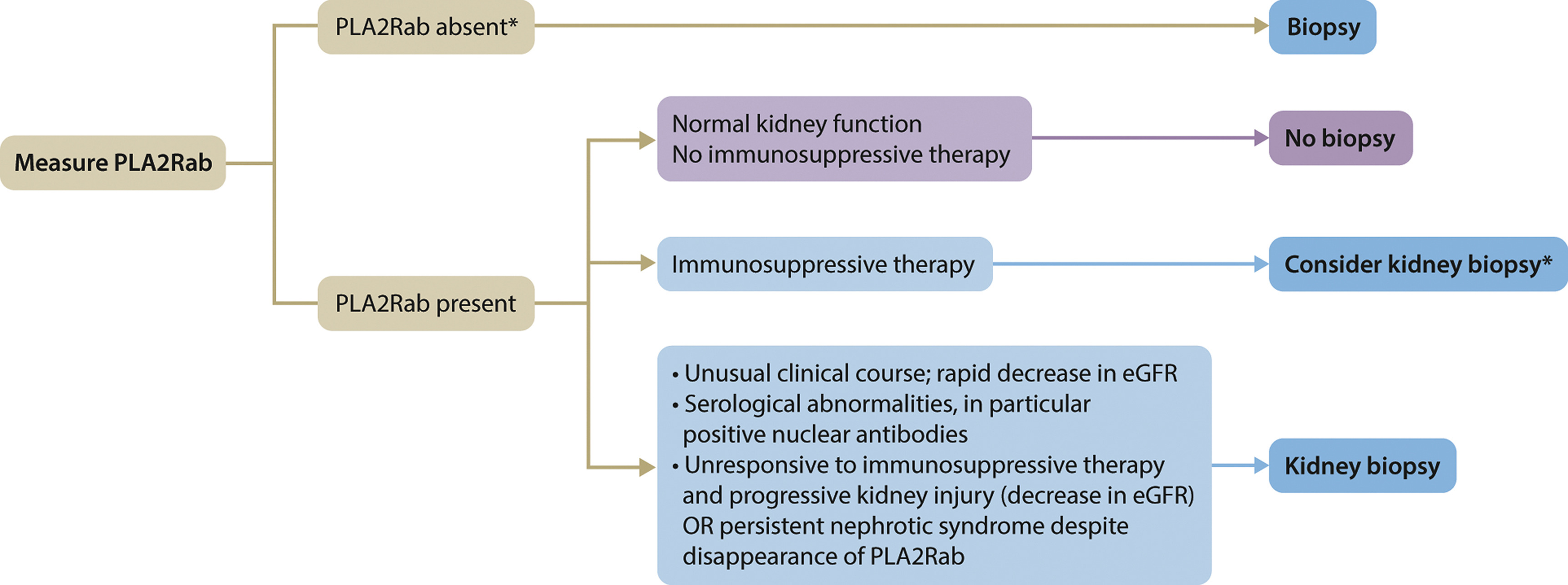
ↈ 何时考虑对抗PLA2R抗体阳性的患者进行肾活检
ↈ Evaluation of patients with MN for associated conditions
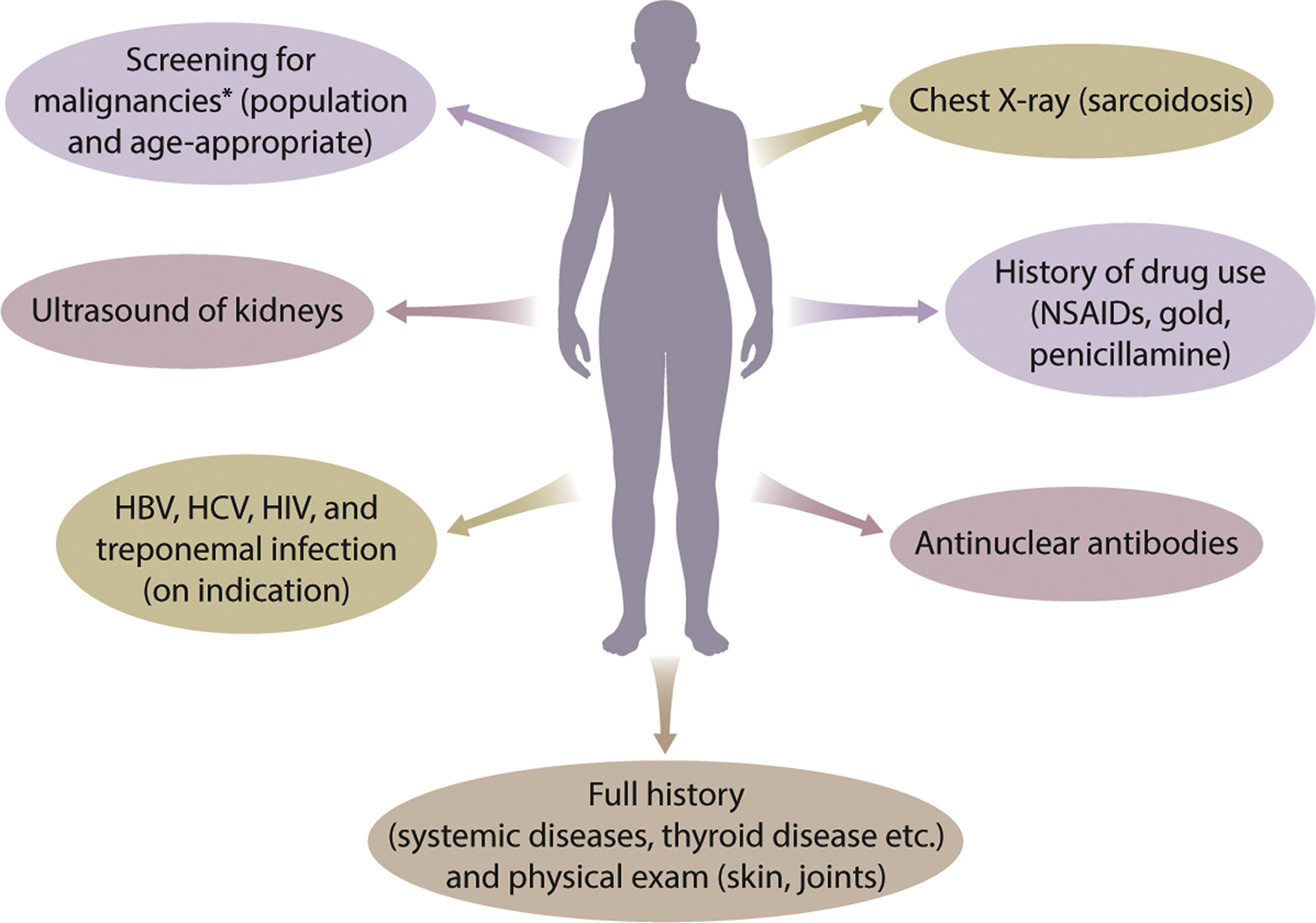
图. In making a decision to perform a kidney biopsy, the risks of a biopsy must be taken into account. The decision is based on patient and physician preferences. This decision to perform a kidney biopsy could be revised in the near future with the development of molecular diagnostics, which could allow for better prediction of outcome for more personalized medicine. eGFR, estimated glomerular filtration rate; PLA2Rab, antibodies against the M-type phospholipase A2 receptor…
ↈ Clinical criteria for assessing risk of progressive loss of kidney function.
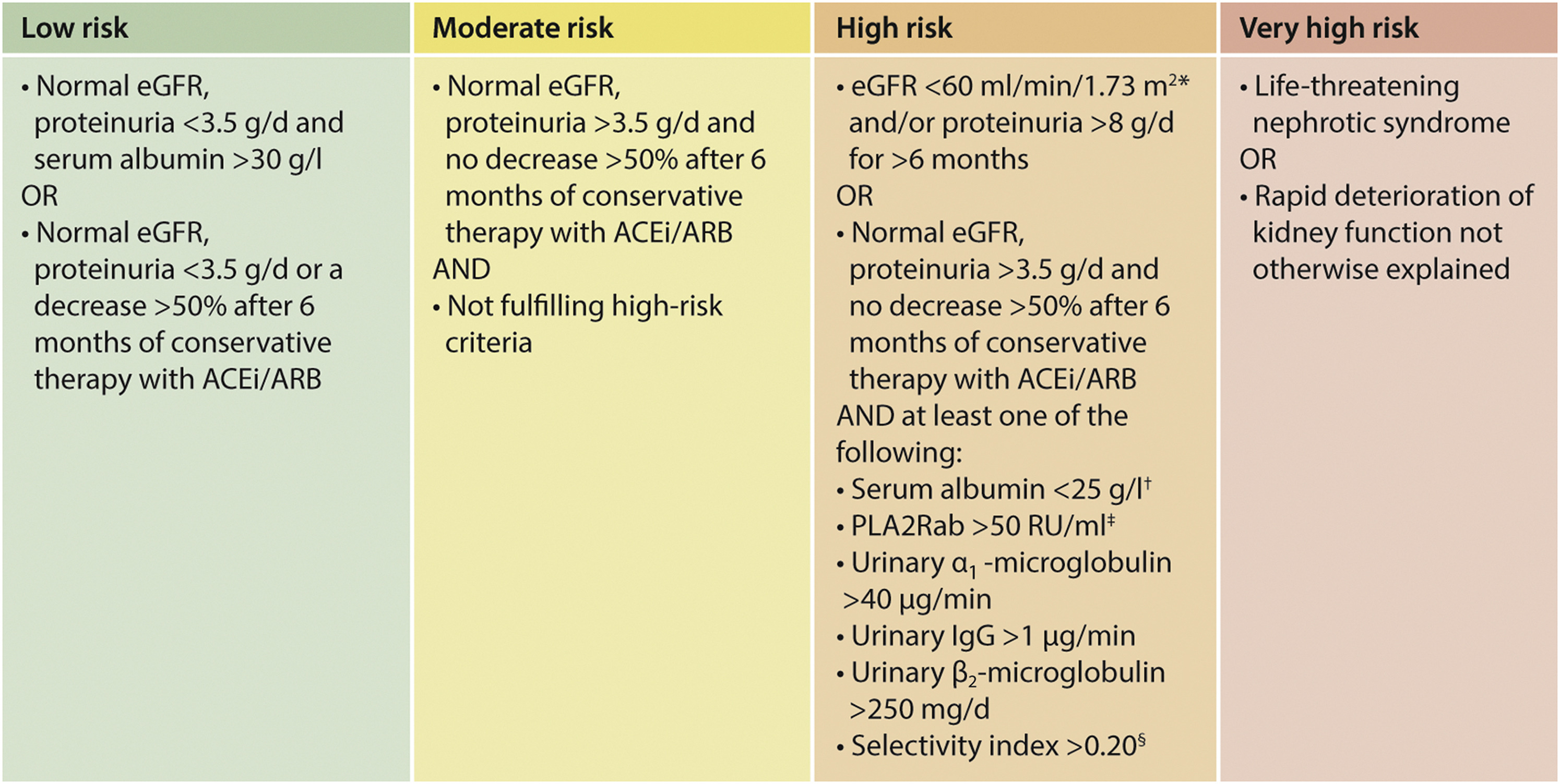
1.大多数研究都使用血清肌酐(SCr)值来指导治疗,SCr值>1.5 mg/dl(133μmol/l)通常用于定义肾功能不全。eGFR值为60ml/min/1.73m2定义了年轻成年人的肾功能不全。
2.eGFR随着年龄的增长而降低,1.5 mg/dl(133μmol/l)的SCr值反映了60岁男性患者的eGFR为50 ml/min/1.73 m2,60岁女性患者为37 ml/min/1.73m2。在风险评估中使用eGFR时,应考虑年龄.
3.抗PLA2R抗体应每隔3至6个月进行一次测量,较短的间隔时间用于基线时抗体水平较高的患者。
4.随访期间抗PLA2R抗体水平的变化可能会增加风险估计。抗PLA2R抗体的消失先于临床缓解,应避免额外治疗。
5. 注意:§选择性指数计算为IgG清除率/白蛋白清除率。
ↈ 膜性肾病的治疗
†钙调神经磷酸酶抑制剂(CNI)单药治疗被认为效率较低。CNI治疗6-12个月并迅速停药与高复发率相关。尽管如此,它的使用可能会被考虑用于eGFR正常且进展风险中等的患者,因为这些患者中的许多人会出现自发缓解。CNI的使用将缩短蛋白尿的时间。对于进展风险高的患者,建议在CNI治疗6个月后添加利妥昔单抗,但CNI治疗后抗PLA2R抗体消失的患者可能除外没有足够的证据表明标准剂量的利妥昔单抗可以预防肾衰竭的发展。
2. 如果eGFR低于50 ml/min/1.73 m2,环磷酰胺的剂量应减半。对于不能耐受或不能再使用环磷酰胺的患者,可以提供利妥昔单抗。
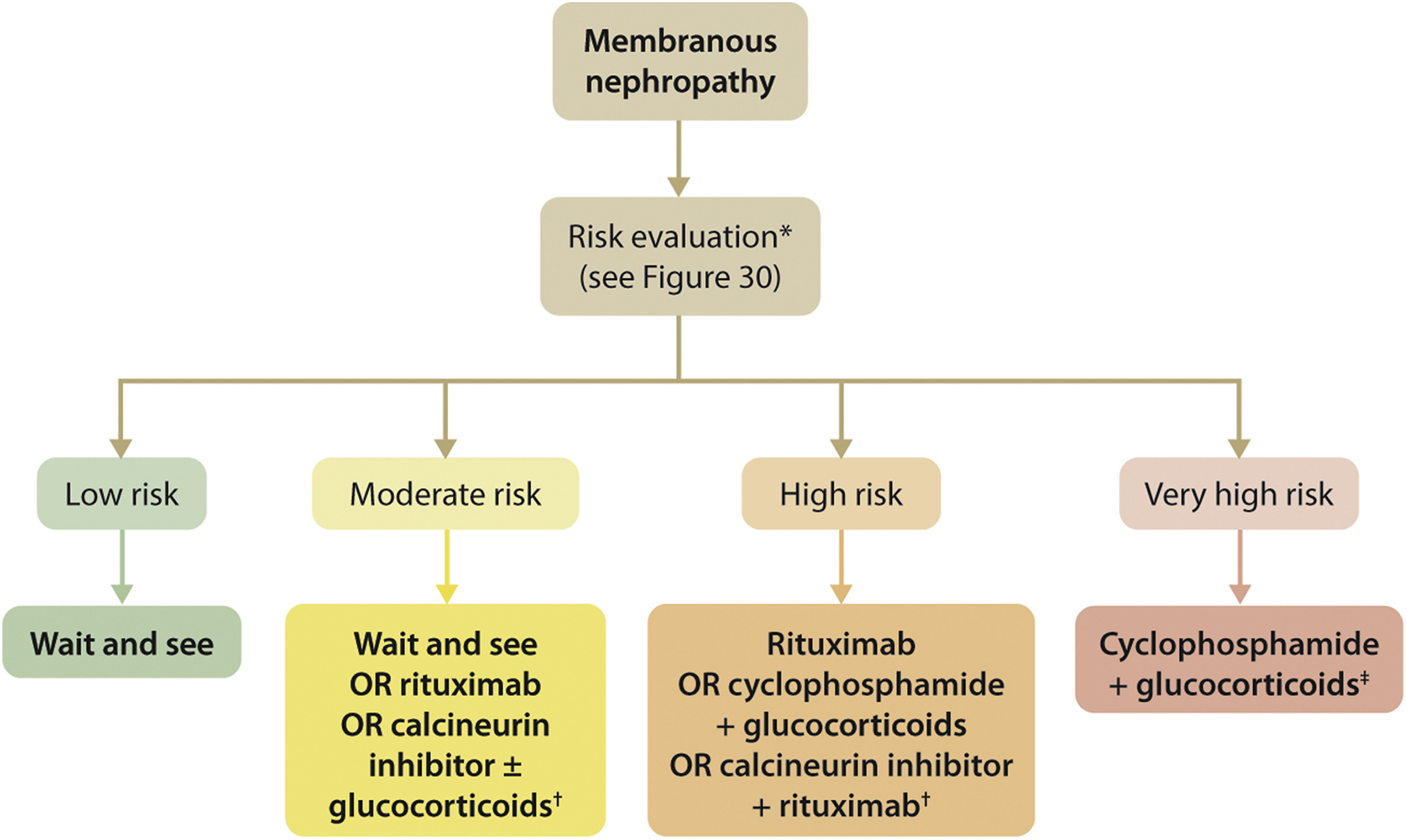
- 推荐意见3.3.1: For patients with MN and at least one risk factor for disease progression, we recommend using rituximab or cyclophosphamide and alternate month glucocorticoids for 6 months, or CNI-based therapy for ≥6 months, with the choice of treatment depending on the risk estimate
- 推荐意见3.3.2: Immunosuppressive therapy is not required in patients with MN, proteinuria ❤️.5 g/d, serum albumin >30 g/l by bromocresol purple (BCP) or immunometric assay, and eGFR >60 ml/min/1.73 m2
- 推荐意见3.3.3: Immunosuppressive therapy is not required in patients with MN, nephrotic syndrome, and normal eGFR, unless at least one risk factor for disease progression is present or serious complications of nephrotic syndrome (e.g., AKI, infections, thromboembolic events) have occurred.
- 推荐意见3.3.4: Longitudinal monitoring of anti-PLA2R antibody levels at 6 months after start of therapy may be useful for evaluating treatment response in patients with MN, and can be used to guide adjustments to therapy
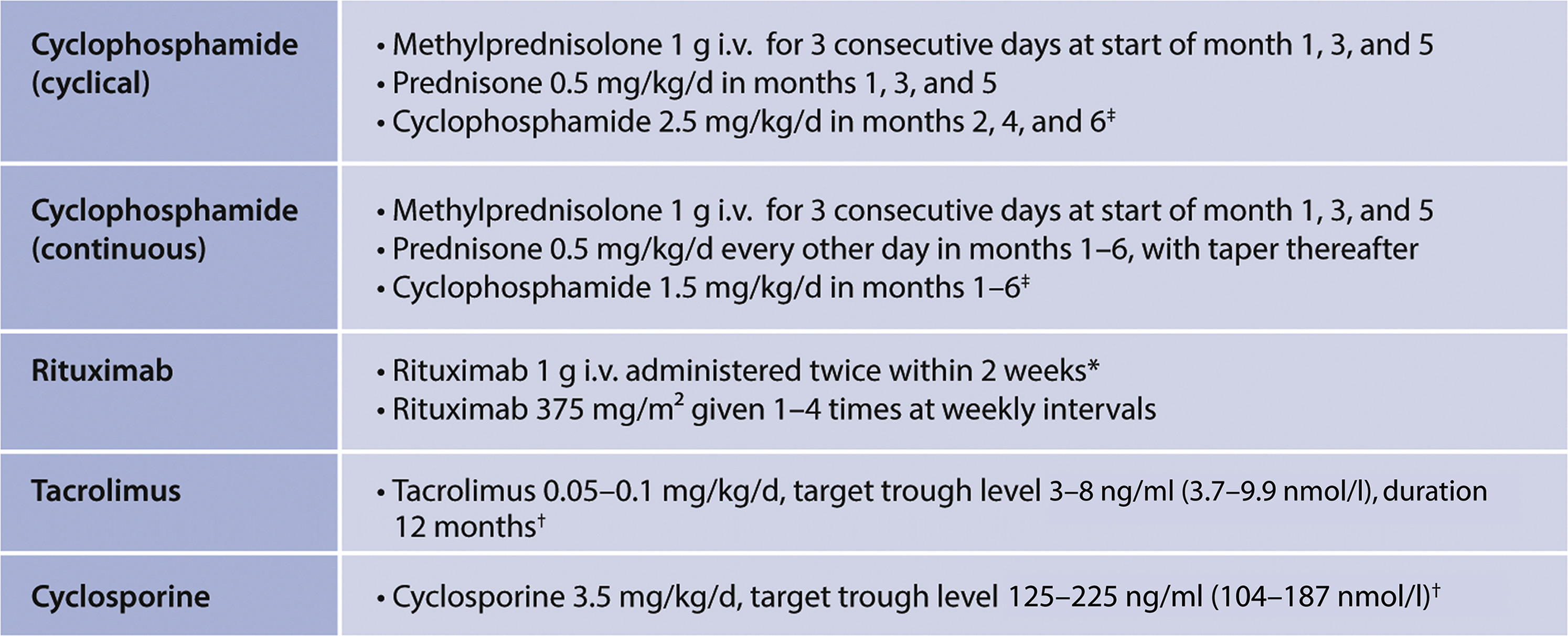 > Figure 32. Commonly used treatment regimens for patients with MN. Mycophenolate mofetil is not discussed. The KDIGO 2012 guideline argued against the use of MMF monotherapy in patients with MN. This still holds and is based on the results of 1 RCT.172 In this study of 36 patients, MMF monotherapy for 12 months did not increase remission rate (37% vs. 41%). MMF in combination with glucocorticoids, is more effective. Small RCTs compared MMF and glucocorticoids with either alkylating agents173,174 or CNI.168,175 In these studies, all with relative short follow-up, remission rates were comparable. A study using historical controls and comparing MMF with cyclophosphamide also reported similar remission rates. However, relapse rate within 24 months of follow-up was markedly higher in MMF-treated patients.176 A more detailed evaluation showed that immunologic remissions were less likely to occur with MMF.177 The dose of MMF could be the most relevant variable; studies in LN have used higher dosages (3 g vs. 2 g), and in patients with SSNS, relapse rate was lower in patients with higher drug concentrations.178 Note: Prednisone and prednisolone are equivalent, used in the same dosage, and have both been used in RCTs, depending on the country of origin. All later usages of “prednisone” in this guideline refer to prednisone or prednisolone. All later usages of “glucocorticoids” refer to prednisone or prednisolone, unless specified otherwise. ‡Recent studies have used i.v. cyclophosphamide. These studies included patients with maintained eGFR. There are no RCTs evaluating the efficacy of i.v. cyclophosphamide on kidney endpoints. Older RCTs using i.v. cyclophosphamide that included patients with deteriorating eGFR were negative.170,171 Intravenous cyclophosphamide might be considered in patients with normal eGFR, in whom the lowest possible cumulative dose of cyclophosphamide should be used (previous use of cyclophosphamide, patients with wishes to bear children) or in countries where p.o. cyclophosphamide is not available. ∗Consider repeating after 6 months in patients with persistent NS, stable eGFR, especially if anti-PLA2R antibodies remained positive. †Cyclosporine and tacrolimus are often given in combination with prednisone in a dose of 10 mg/d. After 4 months, withdrawal if no response; after 12 months, consider tapering to lower levels. There are few trials that have compared the dose and duration of CNI therapy. Yuan et al. compared 6 months versus 24 months of tacrolimus and prednisone.169 Remission rates after 6 months were comparable (18/20 versus 18/22), however persistent remission after 24 months was observed in only 9/18 patients treated for 6 months versus 18/18 patients treated for 24 months. A meta-analysis confirmed high remission and high relapse rates. These findings can be discussed with the patient while agreeing on the duration of therapy. MN, membranous nephropathy; PLA2R, M-type phospholipase A2 receptor.
> Figure 32. Commonly used treatment regimens for patients with MN. Mycophenolate mofetil is not discussed. The KDIGO 2012 guideline argued against the use of MMF monotherapy in patients with MN. This still holds and is based on the results of 1 RCT.172 In this study of 36 patients, MMF monotherapy for 12 months did not increase remission rate (37% vs. 41%). MMF in combination with glucocorticoids, is more effective. Small RCTs compared MMF and glucocorticoids with either alkylating agents173,174 or CNI.168,175 In these studies, all with relative short follow-up, remission rates were comparable. A study using historical controls and comparing MMF with cyclophosphamide also reported similar remission rates. However, relapse rate within 24 months of follow-up was markedly higher in MMF-treated patients.176 A more detailed evaluation showed that immunologic remissions were less likely to occur with MMF.177 The dose of MMF could be the most relevant variable; studies in LN have used higher dosages (3 g vs. 2 g), and in patients with SSNS, relapse rate was lower in patients with higher drug concentrations.178 Note: Prednisone and prednisolone are equivalent, used in the same dosage, and have both been used in RCTs, depending on the country of origin. All later usages of “prednisone” in this guideline refer to prednisone or prednisolone. All later usages of “glucocorticoids” refer to prednisone or prednisolone, unless specified otherwise. ‡Recent studies have used i.v. cyclophosphamide. These studies included patients with maintained eGFR. There are no RCTs evaluating the efficacy of i.v. cyclophosphamide on kidney endpoints. Older RCTs using i.v. cyclophosphamide that included patients with deteriorating eGFR were negative.170,171 Intravenous cyclophosphamide might be considered in patients with normal eGFR, in whom the lowest possible cumulative dose of cyclophosphamide should be used (previous use of cyclophosphamide, patients with wishes to bear children) or in countries where p.o. cyclophosphamide is not available. ∗Consider repeating after 6 months in patients with persistent NS, stable eGFR, especially if anti-PLA2R antibodies remained positive. †Cyclosporine and tacrolimus are often given in combination with prednisone in a dose of 10 mg/d. After 4 months, withdrawal if no response; after 12 months, consider tapering to lower levels. There are few trials that have compared the dose and duration of CNI therapy. Yuan et al. compared 6 months versus 24 months of tacrolimus and prednisone.169 Remission rates after 6 months were comparable (18/20 versus 18/22), however persistent remission after 24 months was observed in only 9/18 patients treated for 6 months versus 18/18 patients treated for 24 months. A meta-analysis confirmed high remission and high relapse rates. These findings can be discussed with the patient while agreeing on the duration of therapy. MN, membranous nephropathy; PLA2R, M-type phospholipase A2 receptor.
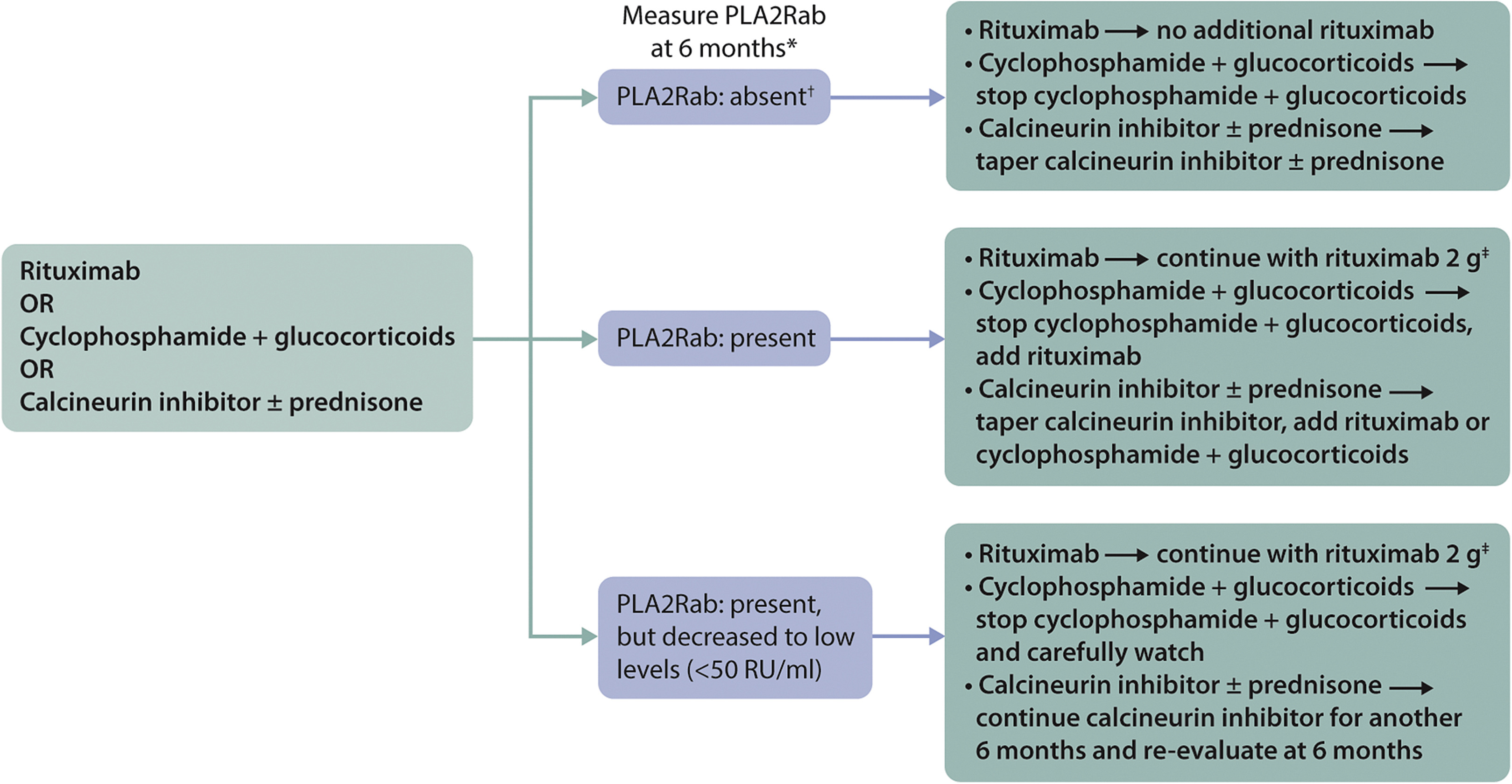
1. 环磷酰胺的累计计量问题:考虑到恶性肿瘤的风险,环磷酰胺的累积剂量不应超过36g。为了安全起见,我们通常将累积剂量限制在25 g(对于80 kg的雄性:6个月周期性环磷酰胺2.5 mg/kg/d相当于18 g,6个月每日环磷酰胺1.5 mg/kg/d等于22 g)。希望怀孕的患者必须使用较低剂量(最多10g)。
2. CNI不太可能诱导晚期免疫缓解,在具有持久性抗PLA2R抗体的患者中,这些药物可以与利妥昔单抗联合使用。
3. B细胞耗竭不足以判断利妥昔单抗治疗的疗效;即使外周血中的B细胞不存在或非常低,也可以考虑额外的剂量。eGFR应维持稳定;如果不是,有必要评估其他原因,如果eGFR下降归因于MN活动,则应提供额外的治疗
4.一些中心将在第3个月测量抗PLA2R抗体,并在那时调整治疗。大多数患者在开始治疗后3个月内出现反应。†免疫荧光试验阴性表明免疫缓解。如果通过酶联免疫吸附测定进行测量,则应使用2 RU/ml的临界值来定义完全免疫缓解利妥昔单抗的再治疗应与初始治疗类似,每2周输注1或2次1g利妥昔mab
- Practice Point 3.4.1: Algorithm for the treatment of patients with MN and initial relapse after therapy
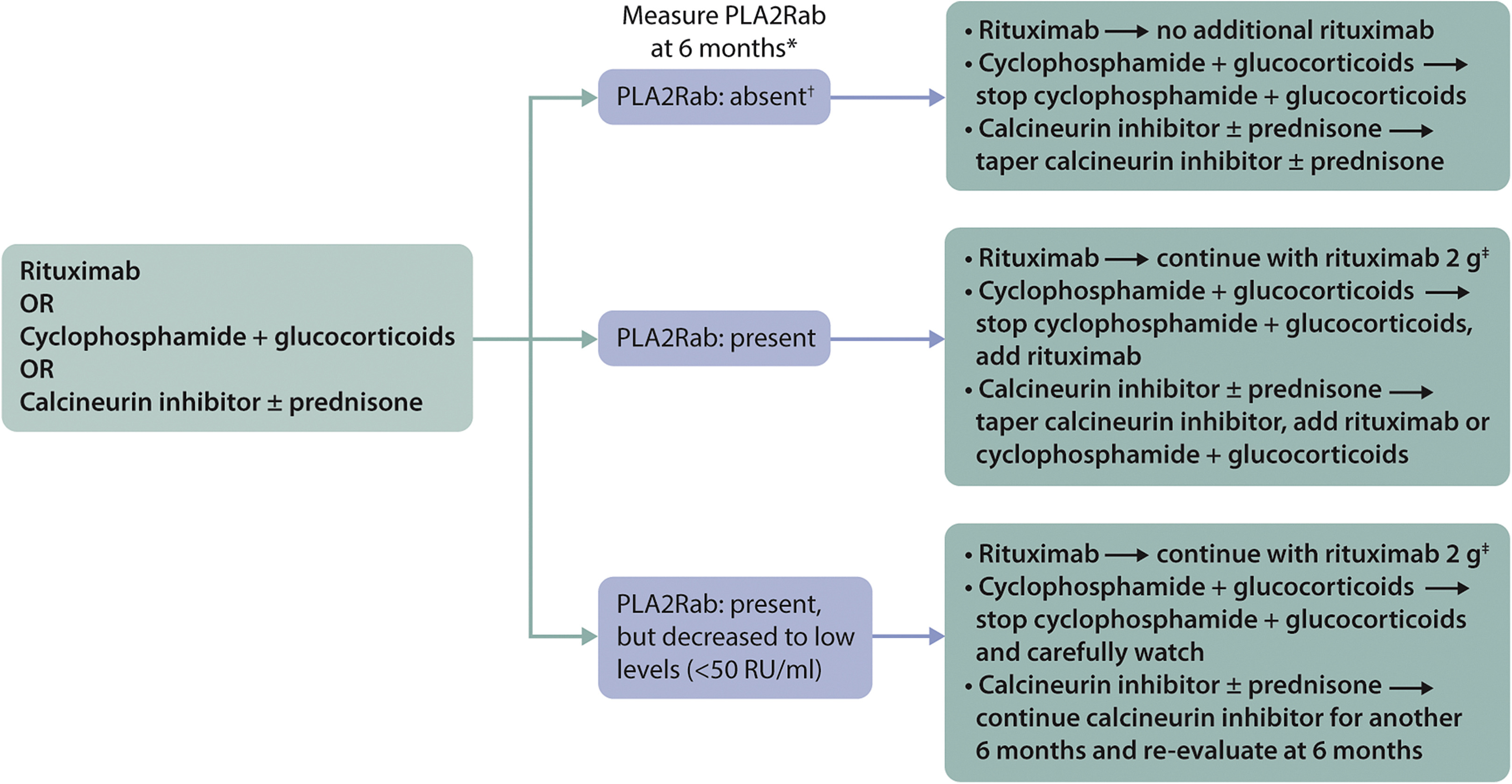
1. 复发的定义: 有些人定义为proteinuria >3.5 g/d in patients who developed a partial or complete remission;建议用ALB和PCR评估:We PCR decreased to values between 2–3.5 g/d without an increase of serum albumin to normal, the subsequent rise in PCR should be considered resistant disease rather than relapse after remission. In patients with a partial remission (characterized by normalization of serum albumin), a relapse should be defined by an increase of proteinuria paralleled by a decrease in serum albumin levels. †Immunologic monitoring is of particularly great value in these situations.
2. 免疫学监测在这些情况下具有特别重要的价值。如果在“临床缓解”期间,抗PLA2R抗体仍然呈阳性,这将是耐药性疾病的证据。因此,在抗PLA2R抗体呈阳性的患者中,建议在病情缓解和复发时评估抗PLA1R抗体。抗PLA2R抗体的过程应先于临床过程。对于早期复发的患者,重要的是要考虑先前治疗失败的原因(例如依从性、药物水平低、B细胞耗竭不足、存在抗利妥昔单抗抗体)
- 推荐意见3.4.2:抵抗患者的治疗
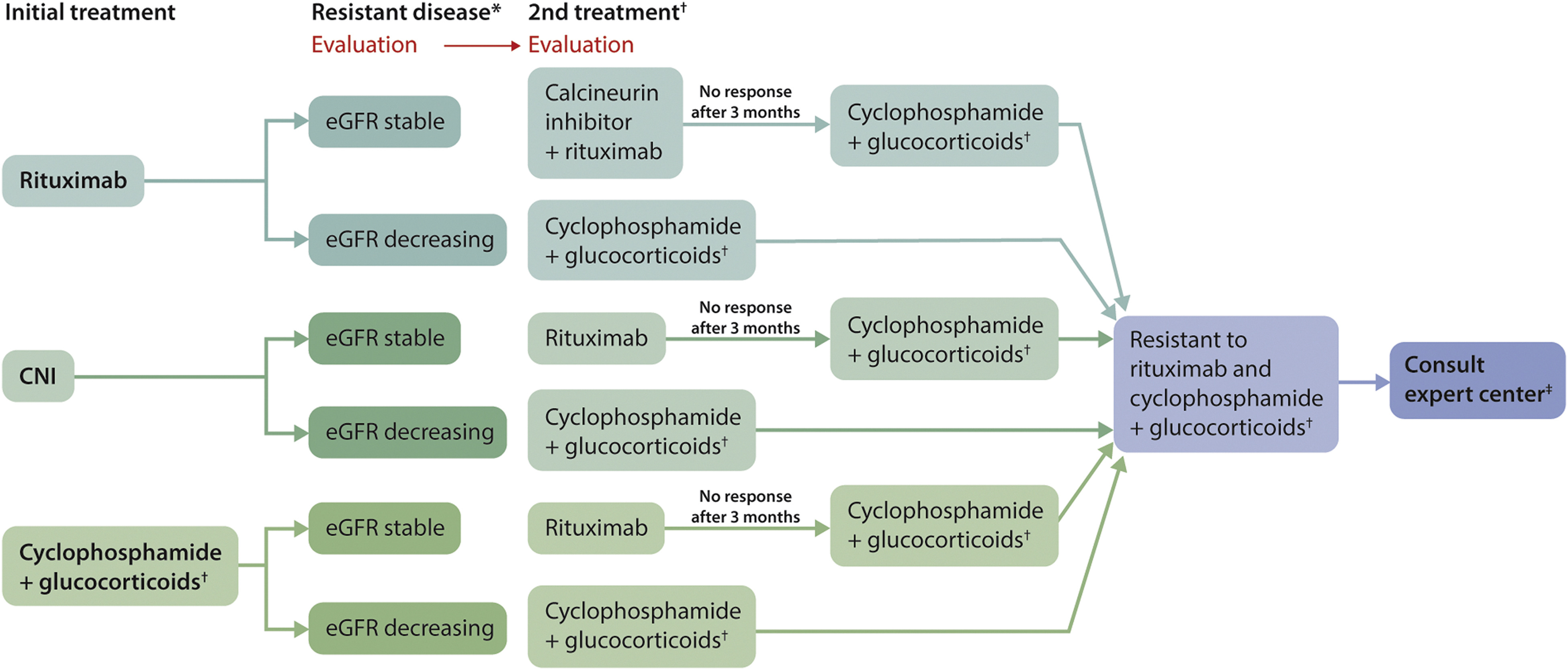
1. 有效性监测:B cell response, anti-rituximab antibodies, IgG levels, leukocytopenia during cyclophosphamide, CNI levels
2.持续的蛋白尿并不能定义抵抗。如果有持续的蛋白尿,但白蛋白升高,考虑可能合并FSCS,anti-PLA2R转阴更支持上述结论。
3.对于有持续蛋白尿,但白蛋白正常或接近正常的患者,尽管PLA2R抗体(-)。需要肾活检considered to document active MN。
Second treatment is dependent on the severity of deterioration of eGFR as indicated. When rituximab is chosen as second treatment, the response of proteinuria and anti-PLA2R antibodies should be evaluated after 3 months. Cyclophosphamide treatment should take into account the maximal tolerable dose: The cumulative dose should not exceed 10 g if preservation of fertility is required. The cumulative dose should not exceed 36 g to limit risk of malignancies. Expert centers may still use more, based on weighing risk and benefits. ‡Patients who did not respond to rituximab or cyclophosphamide should have a consultation with an expert center. These centers may choose experimental therapies (bortezomib, anti-CD38 therapy, and belimumab) or a higher dose of conventional immunosuppressive therapy. CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerlosclerosis; MN, membranous nephropathy; PLA2R, M-type phospholipase A2 receptor.
Practice Point 3.4.3: Evaluation of a kidney transplant recipient with MN (Figure 36)
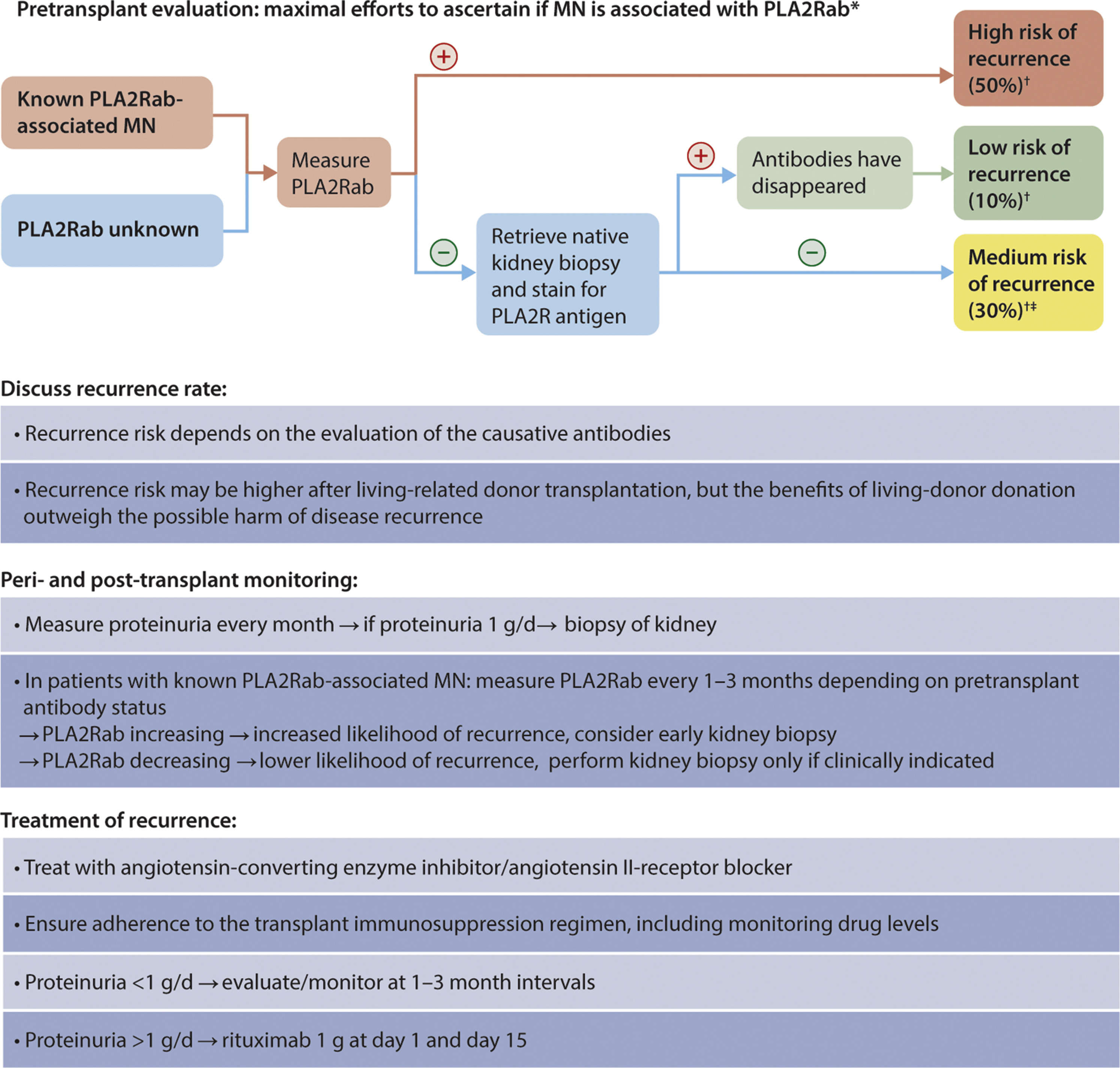
)
Figure 36. Evaluation of a kidney transplant recipient with MN. ∗Limited data available, but the same algorithm likely applies to anti-THSD7A-associated MN. †Clinical recurrence. ‡This is the estimated average recurrence rate for patients with MN and unidentified antigen. We suggest that in these patients the recurrence rate can be better estimated by evaluating the patient for THSD7A antigen/antibodies. MN, membranous nephropathy; PLA2Rab, antibodies against the M-type phospholipase A2 receptor; THSD7A, thrombospondin type-1 domain-containing 7A.
Practice Point 3.4.4: Algorithm for management of children with MN (Figure 37)
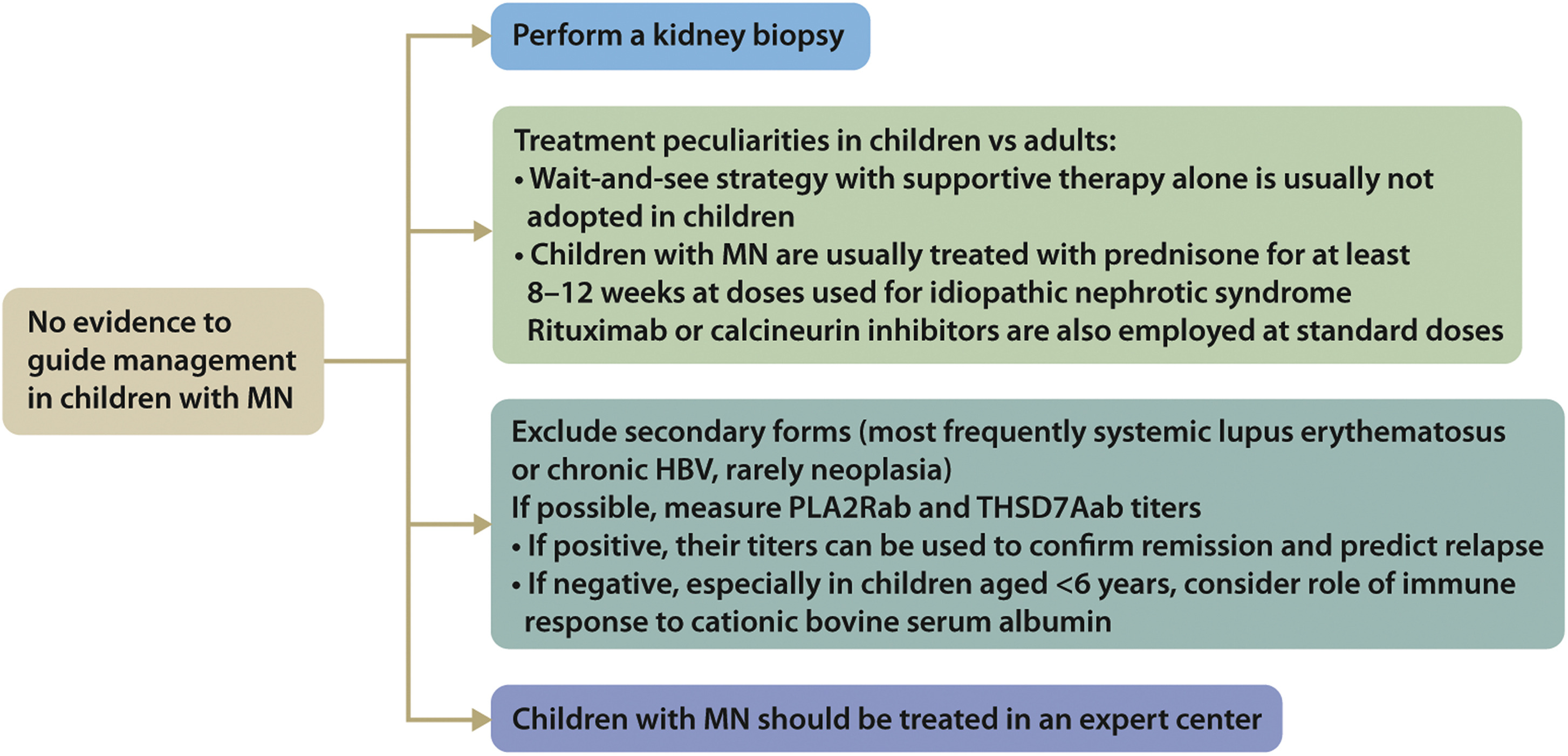
Figure 37. Management of children with MN. HBV, hepatitis B virus; MN, membranous nephropathy; PLA2Rab, antibodies against the M-type phospholipase A2 receptor; THSD7Aab, antibodies against thrombospondin type-1 domain-containing 7A.
Practice Point 3.4.5: Prophylactic anticoagulant therapy in patients with MN and nephrotic syndrome should be based on an estimate of the risk of thrombotic events and the risk of bleeding complications (Figure 38).
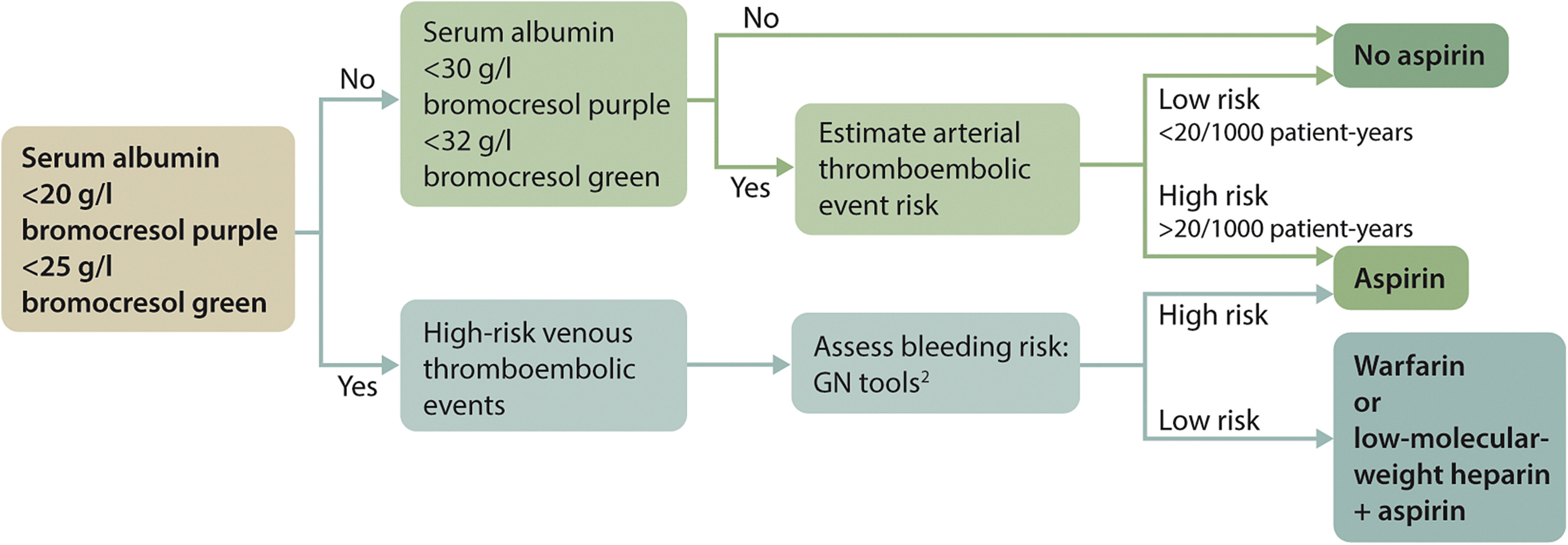
Figure 38. Anticoagulant therapy in patients with MN. Adapted from Kidney International, volume 89, issue 5, Hofstra JM, Wetzels JFM. Should aspirin be used for primary prevention of thrombotic events in patients with membranous nephropathy? Pages 981–983, Copyright Copyright 2016, with permission from the International Society of Nephrology.44 Proposed algorithm for anticoagulant therapy in patients with membranous nephropathy (MN). This algorithm provides guidance for the clinicians. The proposed cutoff values are based on expert opinion. When considering anticoagulant therapy, it is important to balance benefits and risks. The following are important considerations:
- 1.The risk of thrombotic events is related to the level of serum albumin. It is important to note that there is a large difference among the serum albumin assays.204 A serum albumin concentration of 25 g/l (2.5 g/dl) with bromocresol green (BCG) equals a concentration of ∼20 g/l (2.0 g/dl) with bromocresol purple (BCP), or immunonephelometry. It is likely that most studies have used the BCG assay. Consider using 25 g/l (2.5 g/dl) as a threshold when using BCG, and 20 g/l (2.0 g/dl) when using BCP or immunonephelometry.
- 2.Assess risk of venous thrombosis and risk of bleeding (https://www.med.unc.edu/gntools/bleedrisk.html).
- 3.Patients with MN and nephrotic syndrome are also at risk of developing arterial thrombotic events. The risk of arterial thromboembolism (ATE) is dependent on age, history of previous events, diabetes, estimated glomerular filtration rate (eGFR), smoking, and severity of nephrotic syndrome (NS). Risk assessment can be done using the Framingham risk score, and including previous events and proteinuria.44
- 4.Use of aspirin is insufficient to prevent venous thromboembolism (VTE); use of warfarin is sufficient to prevent ATE.
- 5.Treatment with warfarin: There is more international normalized ratio (INR) variability in nephrotic syndrome and low eGFR; there is increased risk of thrombosis immediately after starting high-dose warfarin. Consider starting anticoagulation therapy with low-dose low-molecular-weight heparin and then folding-in warfarin and, when therapeutic, stopping the heparin. A good alternative is to use low-dose low-molecular-weight heparin + aspirin for a period of 3 months before switching to warfarin, allowing for judgment on the course of proteinuria.205
- 6.Glucocorticoids increase the risk of thrombosis; thus, anticoagulant therapy should not be omitted in patients who start prednisone therapy.
- 7.ATE risk is estimated using the Framingham risk score, with added risk in case of low eGFR or higher proteinuria. The Framingham risk score takes into account age, smoking, serum cholesterol, and blood pressure.
- Practice Point 4.1.1: The definitions relating to nephrotic syndrome in children are based on the clinical characteristics outlined in Figure 39206.
 > Figure 39. Definitions relating to NS in children aged 1–18 years. ∗To rule out orthostatic proteinuria, the first morning urine should be collected separately for assessment. †van der Watt et al.206 NS, nephrotic syndrome; PCR, protein-creatinine ratio; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome
> Figure 39. Definitions relating to NS in children aged 1–18 years. ∗To rule out orthostatic proteinuria, the first morning urine should be collected separately for assessment. †van der Watt et al.206 NS, nephrotic syndrome; PCR, protein-creatinine ratio; SRNS, steroid-resistant nephrotic syndrome; SSNS, steroid-sensitive nephrotic syndrome
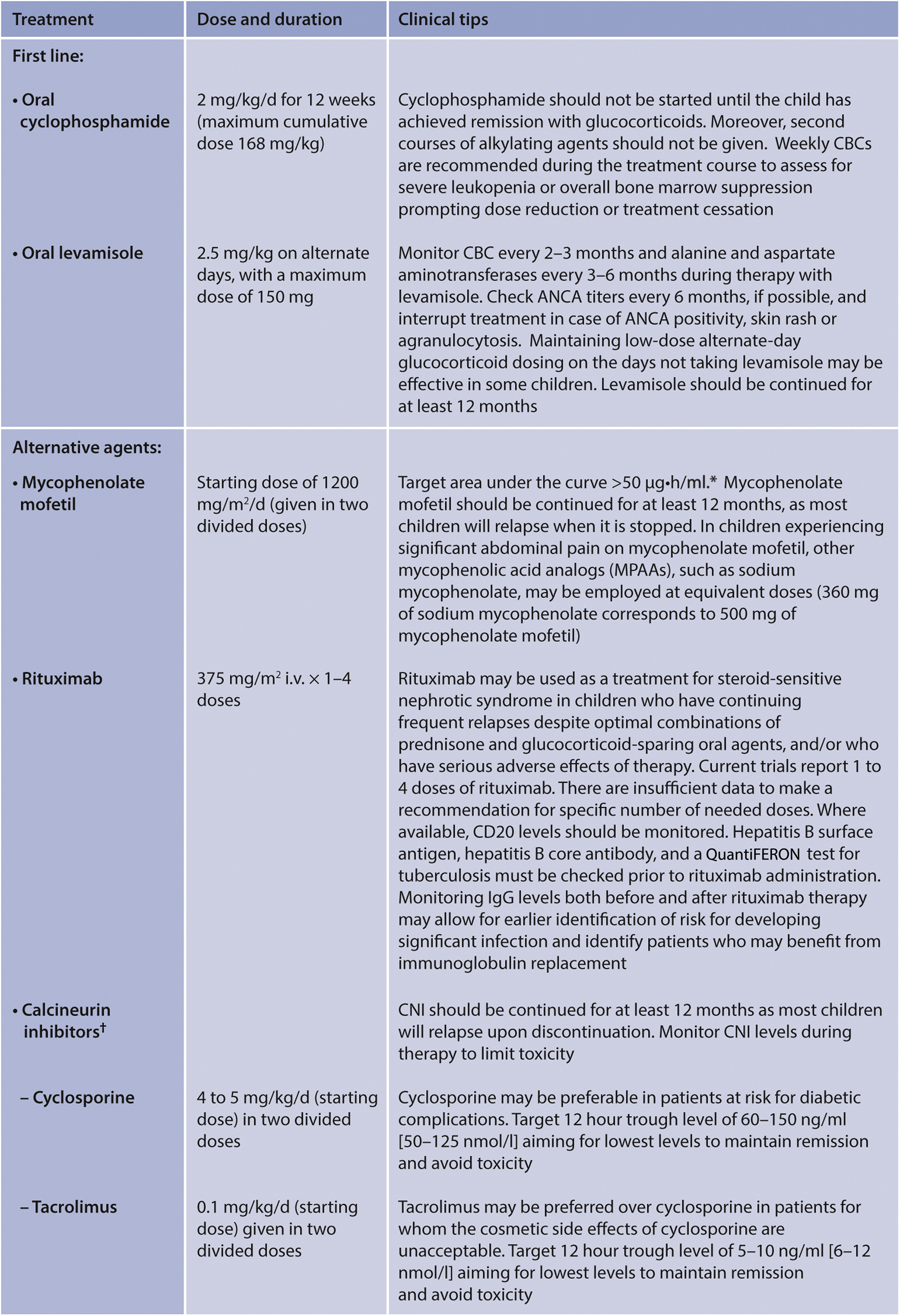
Figure 41. Glucocorticoid-sparing therapies in children with SSNS. ∗Gellermann et al.178 †The CNI, while often used twice daily, may be dosed once a day, depending on individual formulations. In smaller children (<6 years of age), daily dose of cyclosporine can be divided into 3 doses (every 8 hour) to obtain steady hematic levels. Blood levels of CNI do not provide information on intracellular levels. The target ranges for CNIs have been based on the transplant literature. The KDIGO Work Group acknowledges that targets for glomerular diseases are not known. Most clinicians check these levels to verify adherence and avoid CNI toxicity. At present, the most reasonable dosing of a CNI may be to titrate in the individual patient to obtain the desired effect on proteinuria, balancing dose escalation against serum creatinine and reducing the dose if serum creatinine increases but does not plateau or increases over 30% of baseline. If the serum creatinine level does not fall after dose reduction, the CNI should be discontinued. ANCA, antineutrophil cytoplasmic antibody; CBC, complete blood count; CNI, calcineurin inhibitor.























 468
468

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








