前 言
非酒精性脂肪性肝炎(NASH)是一种与肥胖、血脂异常、2型糖尿病和代谢综合征密切相关的疾病,可能会发展为肝硬化、终末期肝病甚至肝癌。据美国肝脏基金会统计数据显示,截至2023年8月,美国成年人中有5%的NASH患者(而NAFLD患者高达25%),且FDA尚未批准任何用于NASH临床治疗的药物。由于NASH发病机制复杂,药物研发道路曲折漫长,主要集中FASN、THR-β、NR1H4/FXR、GLP-1R、PPARA、FGF19、FGF21、ACLY等靶点。
01 NASH基本概念
非酒精性脂肪性肝病(NAFLD)是指除酒精和其他明确损肝因素外,肝细胞内脂肪过度沉积或代谢紊乱引起的慢性肝病,包括单纯性脂肪肝(NAFL)、非酒精性脂肪性肝炎(NASH)、肝纤维化及肝硬化。2022年9月,柳叶刀子刊发表的一项针对全球NAFLD流行病学系统性回顾和荟萃分析显示,全球成人NAFLD患病率约为32.4%。换句话说,全球平均每3个人中就有1人罹患NAFLD。
非酒精性脂肪性肝炎(NASH)是以脂肪在肝脏的过度积累和炎症为特征,可由多种因素导致。大约有15%-25%的NAFLD患者会发展为NASH。NASH一旦发展为晚期,就会引起肝细胞增殖丧失及再生能力下降,进而引发炎症和肝纤维化,并最终可能导致肝硬化、肝功能衰竭,甚至发展为肝癌。
02 NASH治疗靶点研究
对NASH生理和病理机制进行全面的研究,寻找潜在的治疗靶点和生物标志物,对开发有效的干预措施和治疗方案具有重要意义。目前研究表明,FASN、THR-β、NR1H4/FXR、GLP-1R、PPARA、FGF19、FGF21、ACLY等靶点的在体内的失衡与NASH紧密相关。
根据Evaluate Pharm和Frost & Sullivan的评估,到2025年全球NASH药物市场预计将达到350-400亿美元。Madrigal公司的THR-β激动剂(Resmetirom)治疗NASH的III期临床达到主要终点和关键次要终点,可能会成为首款获FDA批准上市的NASH药物。Akero公布的FGF21类似物(Efruxifermin)结果显示,在IIb期临床试验中可改善41%受试者肝纤维化程度,刷新了NASH新药有效性数据。还有今年在减肥领域大火的Retatrutide(GIP/GLP-1/GCG三受体激动剂)在NASH方面的II期临床数据让人感到惊讶,在98名NAFLD患者中肝脏脂肪显著减少,且NASH相关生物标志物显著改善。
| NASH相关治疗靶点与在研药物 | |||
| 靶点 | 作用机制 | 在研药物 | 药理机制 |
| FASN | 失调导致脂肪变性,影响脂代谢,是NASH发生病变的一个重要标志 | FASN抑制剂 | 减少肝脏脂肪积累并减轻炎症 |
| THR-β | 信号传导失调破坏代谢平衡,导致脂肪积累和发生炎症 | THR-β激动剂(Resmetirom) | 调控代谢稳态并改善脂肪变性 |
| NR1H4/FXR | 失调导致胆汁酸介导的肝损伤及炎症反应 | NR1H4/FXR激动剂(Tropifexor、Nidufexor) | 调节胆汁酸代谢和炎症反应 |
| GLP-1R | 功能障碍可能导致胰岛素抗性及脂肪变性 | GLP-1R激动剂(Semaglutide、Liraglutide) | 提高胰岛素敏感性和减少肝脏脂肪变性 |
| PPARA | 信号传导改变导致脂肪代谢受损和加剧炎症 | PPARA激动剂 | 改善脂质代谢和炎症 |
| FGF19、FGF21 | 参与NASH病变肝胆汁酸调节和能量代谢 | FGF19类似物(Aldafermin)、FGF21类似物(Efruxifermin)、FGF21受体激动剂(Pegbelfermin) | 减少脂肪变性和改善肝酶活性 |
| ACLY | 促进脂肪积累 | ACLY抑制剂(Bempedoic) | 预防脂肪肝变性 |
来源:参考文献1-15
03 NASH靶点在研究中的应用
重组靶点蛋白已用于NASH及其他疾病的药物发现和开发研究。Guo团队利用重组人GLP-1受体蛋白(货号:13944-H02H,义翘神州)发现并表征了一种新型GLP-1类似物Ecnoglutide。Qiao团队使用FGF19重组蛋白(货号:12226-HNAE,义翘神州)处理HepG2细胞,证实FGFR4介导FRS2α-ERK通路,为靶向FGFR4治疗肝细胞癌提供理论证据。Hu团队利用FGF19重组蛋白(义翘神州)进行细胞增殖和侵袭实验,发现在晚期浆液性卵巢癌中,发现FGF19-FGFR4信号通路可促进卵巢癌增殖和侵袭。[a1] Lee团队将人FGF21重组蛋白(义翘神州)注射到高血糖小鼠模型,发现FOXO1抑制剂和FGF21联合治疗可提高胰岛素敏感性。
用于表面等离子体共振SPR检测

结果显示GLP-1肽类似物(M2和M4)与人GLP-1R重组蛋白(货号:13944-H02H,义翘神州)的结合亲和力比GLP-1R激动剂(Semaglutide)的高10-30倍。(doi: 10.1016/j.molmet.2023.101762)
用于免疫印迹WB检测
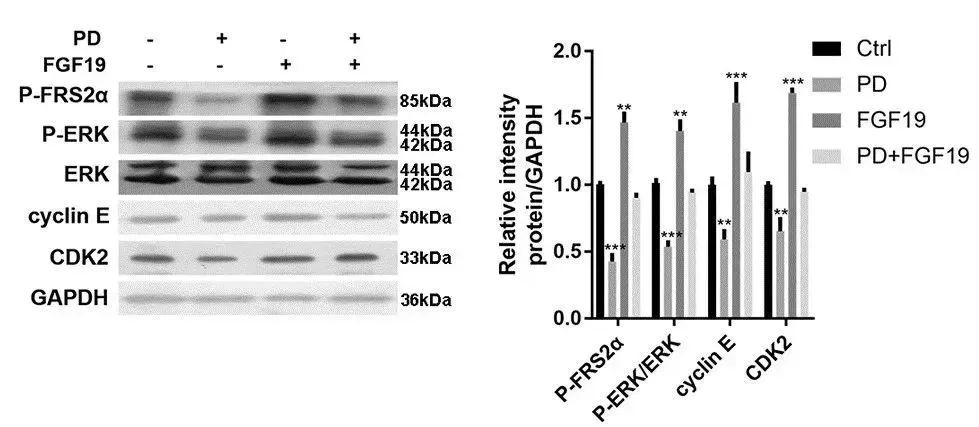
利用FGF19重组蛋白(货号:12226-HNAE,义翘神州)或PD(FGFR4抑制剂)处理HepG2细胞,结果发现FGF19提高P-FRS2α、P-ERK、cyclin E和CDK2表达水平,而PD降低这些蛋白的表达,表明PD能阻断FGFR介导的FRS2α-ERK信号通路,影响肝细胞癌的增殖。(doi: 10.1371/journal.pone.0234708)
用于细胞增殖和侵袭检测
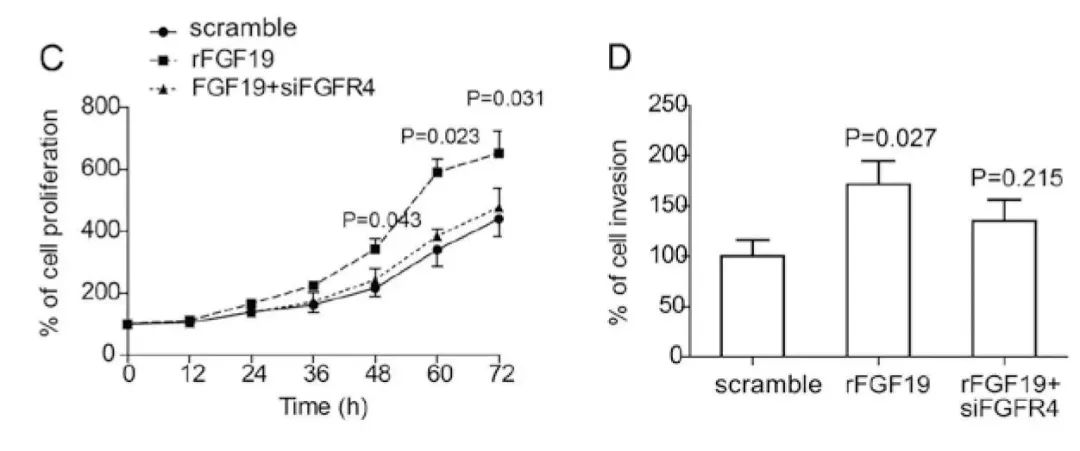
FGF19通过刺激FGFR4促进卵巢癌的进展。(C)rFGF19重组蛋白(购自义翘神州)促进卵巢癌OVCAR3细胞增殖,而FGFR4基因敲除会抑制FGF19诱导的OVCAR3细胞增殖。(D)rFGF19重组蛋白诱导的OVCAR3细胞侵袭需要FGFR4。(doi: 10.3892/or.2015.4212)
用于小鼠模型静脉注射检测
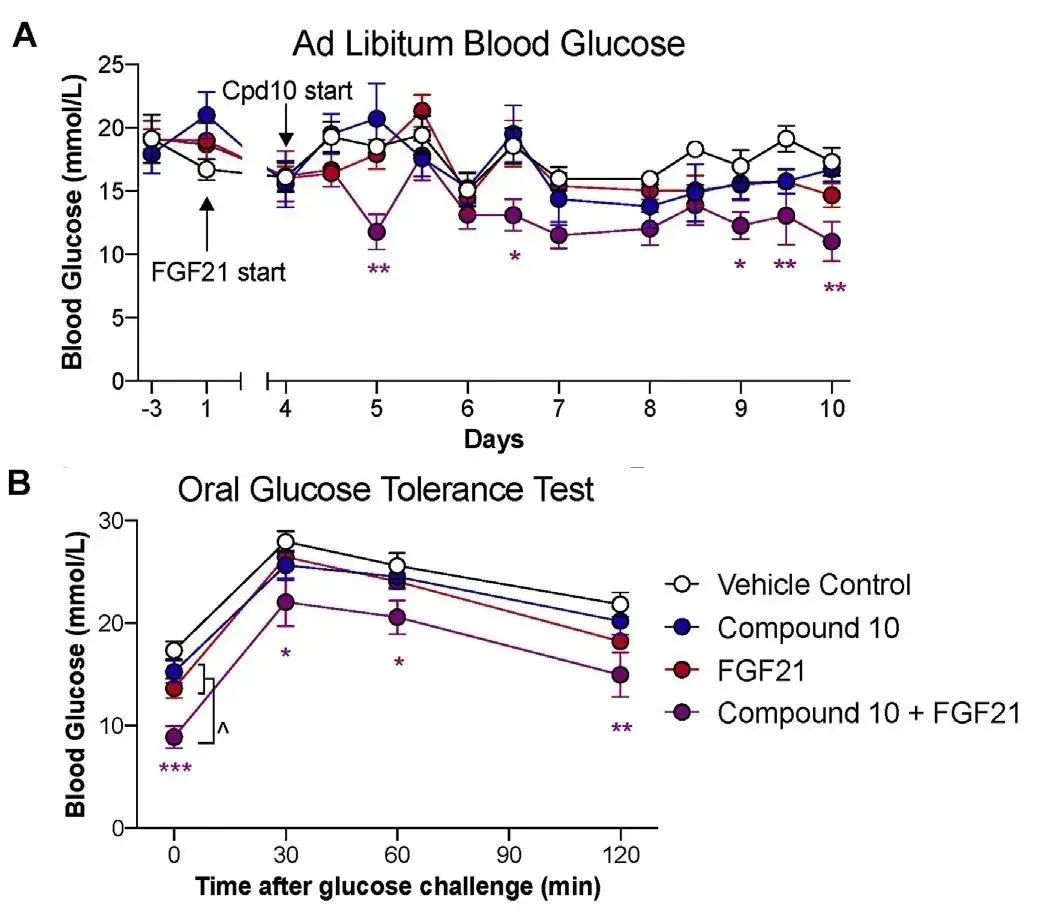
STZ诱导的糖尿病小鼠模型分别接受空白对照组、Compound 10、FGF21重组蛋白(购自义翘神州)及联合治疗(Compound 10和FGF21),(A)用药1小时后测量自由血糖浓度,(B)11天后通过口服葡萄糖耐量试验(OGTT)测定血糖水平。(doi: 10.1016/j.molmet.2021.101187.)
✦义翘神州NASH靶点明星产品
高纯度:
THR-β Protein, Human, Recombinant (His Tag), HPLC-verified, Cat: 15737-H07E
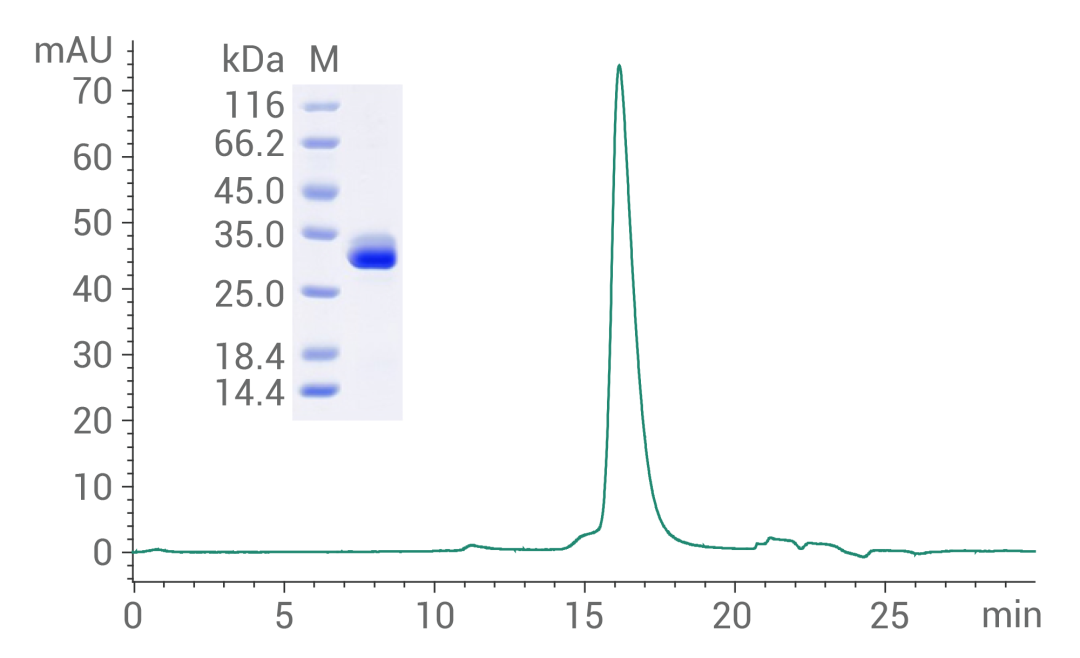
Purity ≥ 95 % as determined by SDS-PAGE,
≥ 90 % as determined by SEC-HPLC.
GLP-1R Protein, Human, Recombinant (His & AVI Tag), HPLC-verified,Cat: 13944-H49H-B

Purity ≥ 95 % as determined by SDS-PAGE,
≥ 95 % as determined by SEC-HPLC.
| 更多NASH靶点明星产品 | |||
| 货号 | 靶点 | 种属 | 纯度/标签 |
| ACLY | Human | >85%、His | |
| GLP-1R | Human | >95%、hFc | |
| FASN | Human | >95%、His | |
| FASN | Mouse | >95%、His | |
| NR1H4 | Mouse | >95%、His | |
| PPARA | Human | >90%、His | |
| PPARA | Mouse | >90%、His | |
| FGF19 | Human | >90% | |
| FGF21 | Human | >95%、His | |
| FGF21 | Mouse | >85%、His | |
| FGF21 | Human | >95% | |
| FGF21 | Hamster | >95%、His | |
注:蛋白纯度均为SDS-PAGE检测结果
【参考文献】
1. Cotter, T. G. & Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 158, 1851–1864 (2020).
2. Kiarash Riazi, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol, 2022. DOI: 10.1016/S2468-1253(22)00165-0
3. NASH Definition & Prevalence - American Liver Foundation. https://liverfoundation.org/liver-diseases/fatty-liver-disease/nonalcoholic-steatohepatitis-nash/nash-definition-prevalence/.
4. van de Wiel, et al. Identification of FDA-approved drugs targeting the Farnesoid X Receptor. Sci Rep 9, (2019).
5. Xu, X. et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduction and Targeted Therapy vol. 7 Preprint at https://doi.org/10.1038/s41392-022-01119-3 (2022).
6. Sanyal, A. J. et al. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med 29, 392–400 (2023).
7. Feng, X., et al. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog Lipid Res 77, 101006 (2020).
8. Henriksson, E. & Andersen, B. FGF19 and FGF21 for the Treatment of NASH—Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human. Frontiers in Endocrinology vol. 11 Preprint at https://doi.org/10.3389/fendo.2020.601349 (2020).
9. Morrow, M. R. et al. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab 34, 919-936.e8 (2022).
10. Saponaro, F., Sestito, S., Runfola, M., Rapposelli, S. & Chiellini, G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Frontiers in Medicine vol. 7 Preprint at https://doi.org/10.3389/fmed.2020.00331 (2020).
11. Newsome, P. N. et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. New England Journal of Medicine 384, 1113–1124 (2021).
12. Francque, S. M. et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. New England Journal of Medicine 385, 1547–1558 (2021).
13. Lee, Y. K. et al. FOXO1 inhibition synergizes with FGF21 to normalize glucose control in diabetic mice. Mol Metab 49, (2021).
14. Hu, L. & Cong, L. Fibroblast growth factor 19 is correlated with an unfavorable prognosis and promotes progression by activating fibroblast growth factor receptor 4 in advanced-stage serous ovarian cancer. Oncol Rep 34, 2683–2691 (2015).
15. NASH drugs race to cross the finish line - Pharmaceutical Technology. https://www.pharmaceutical-technology.com/features/nash-drugs-race-to-cross-the-finish-line/.
16. Jensen-Urstad, A. P. L. & Semenkovich, C. F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1821, 747–753 (2012).
17. Qiao, C. et al. PD173074 blocks G1/S transition via CUL3-mediated ubiquitin protease in HepG2 and Hep3B cells. PLoS One 15, (2020).
18. Girdhar, K. et al. Design, synthesis, and biological evaluation of a small molecule oral agonist of the glucagon-like-peptide-1 receptor. Journal of Biological Chemistry 298, (2022).
19. Guo, W. et al. Discovery of ecnoglutide – A novel, long-acting, cAMP-biased glucagon-like peptide-1 (GLP-1) analog. Mol Metab 101762 (2023) doi:10.1016/j.molmet.2023.101762.





















 2021
2021

 被折叠的 条评论
为什么被折叠?
被折叠的 条评论
为什么被折叠?








